Plant-mediated RNAi silences midgut-expressed genes in congeneric lepidopteran insects in nature
- PMID: 29132300
- PMCID: PMC5683459
- DOI: 10.1186/s12870-017-1149-5
Plant-mediated RNAi silences midgut-expressed genes in congeneric lepidopteran insects in nature
Abstract
Background: Plant-mediated RNAi (PMRi) silencing of insect genes has enormous potential for crop protection, but whether it works robustly under field conditions, particularly with lepidopteran pests, remains controversial. Wild tobacco Nicotiana attenuata and cultivated tobacco (N. tabacum) (Solanaceae) is attacked by two closely related specialist herbivores Manduca sexta and M. quinquemaculata (Lepidoptera, Sphingidae). When M. sexta larvae attack transgenic N. attenuata plants expressing double-stranded RNA(dsRNA) targeting M. sexta's midgut-expressed genes, the nicotine-ingestion induced cytochrome P450 monooxygenase (invert repeat (ir)CYP6B46-plants) and the lyciumoside-IV-ingestion induced β-glucosidase1 (irBG1-plants), these larval genes which are important for the larvae's response to ingested host toxins, are strongly silenced.
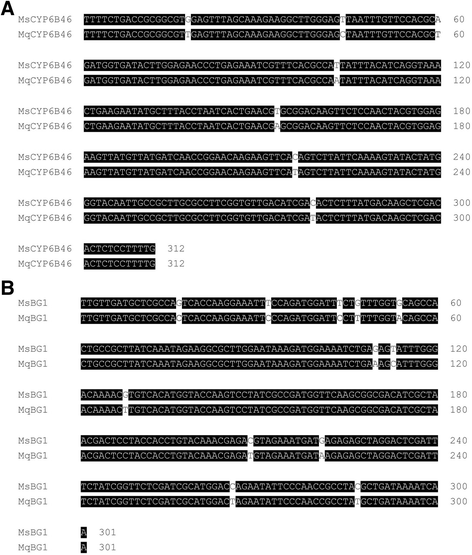
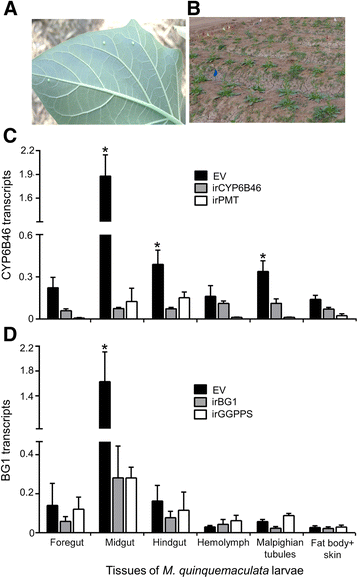
Results: Here we show that the PMRi procedure also silences the homologous genes in native M. quinquemaculata larvae feeding on irCYP6B46 and irBG1-transgenic N. attenuata plants in nature. The PMRi lines shared 98 and 96% sequence similarity with M. quinquemaculata homologous coding sequences, and CYP6B46 and BG1 transcripts were reduced by ca. 90 and 80%, without reducing the transcripts of the larvae's most similar, potential off-target genes.
Conclusions: We conclude that the PMRi procedure can robustly and specifically silence genes in native congeneric insects that share sufficient sequence similarity and with the careful selection of targets, might protect crops from attack by congeneric-groups of insect pests.
Keywords: CYP6B46; Manduca quinquemaculata; Plant-mediated RNAi; RNA interference; Transgenic tobacco plants; β-glucosidase.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, Albrechtsen M, An C, Aymeric JL, Barthel A, et al. RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol. 2011;57(2):231–245. doi: 10.1016/j.jinsphys.2010.11.006. - DOI - PubMed
-
- Liuqi G, Douglas CK. Recent advances in RNA interference research in insects: Implications for future insect pest management strategies. Crop Prot. 2013;45:36–40.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

