Knockdown of SOX2OT inhibits the malignant biological behaviors of glioblastoma stem cells via up-regulating the expression of miR-194-5p and miR-122
- PMID: 29132362
- PMCID: PMC5683208
- DOI: 10.1186/s12943-017-0737-1
Knockdown of SOX2OT inhibits the malignant biological behaviors of glioblastoma stem cells via up-regulating the expression of miR-194-5p and miR-122
Erratum in
-
Correction: Knockdown of SOX2OT inhibits the malignant biological behaviors of glioblastoma stem cells via up-regulating the expression of miR-194-5p and miR-122.Mol Cancer. 2022 Oct 22;21(1):202. doi: 10.1186/s12943-022-01673-y. Mol Cancer. 2022. PMID: 36273152 Free PMC article. No abstract available.
Abstract
Background: Accumulating evidence has highlighted the potential role of long non-coding RNAs (lncRNAs) in the biological behaviors of glioblastoma stem cells (GSCs). Here, we elucidated the function and possible molecular mechanisms of the effect of lncRNA-SOX2OT on the biological behaviors of GSCs.
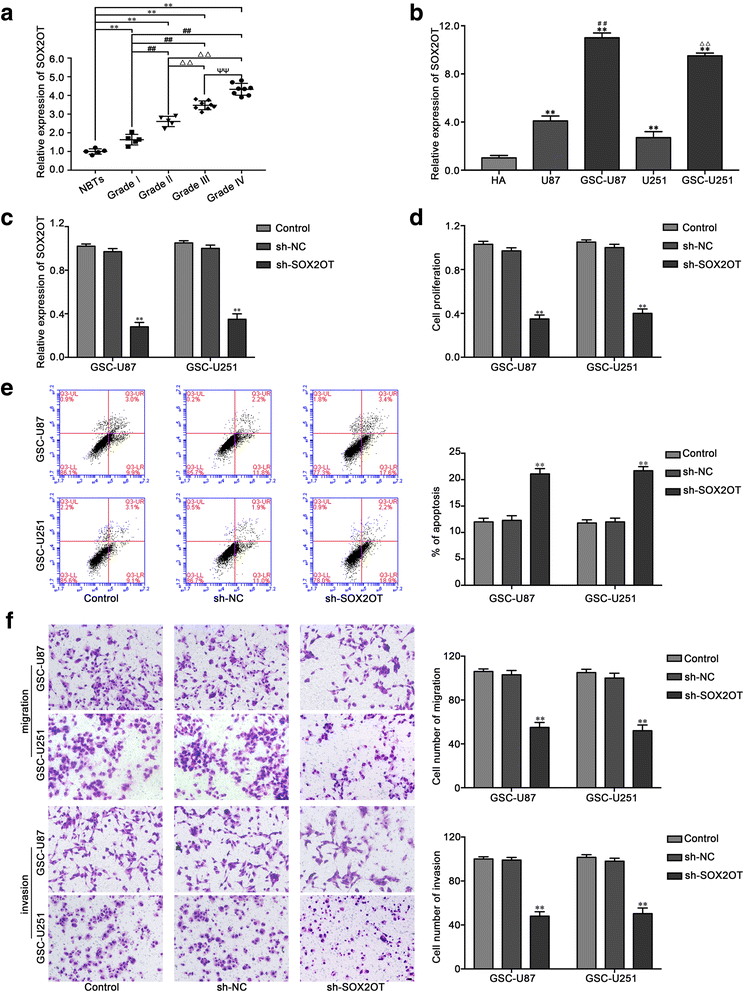
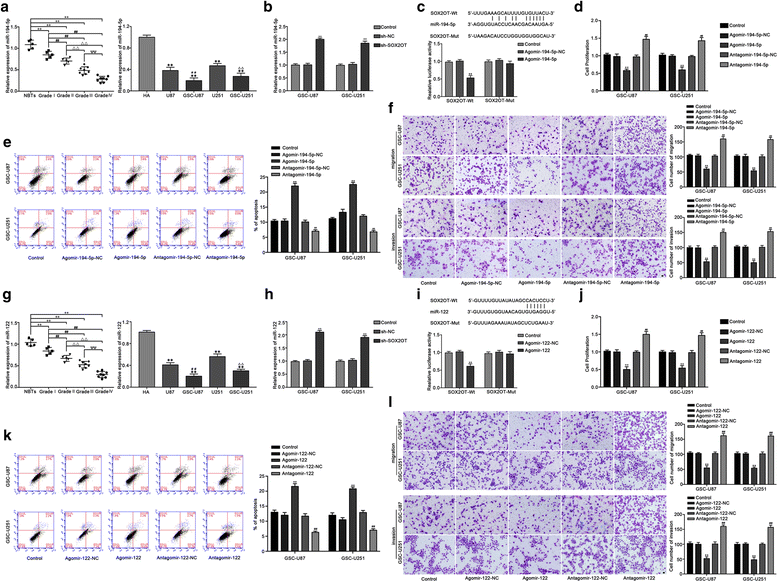
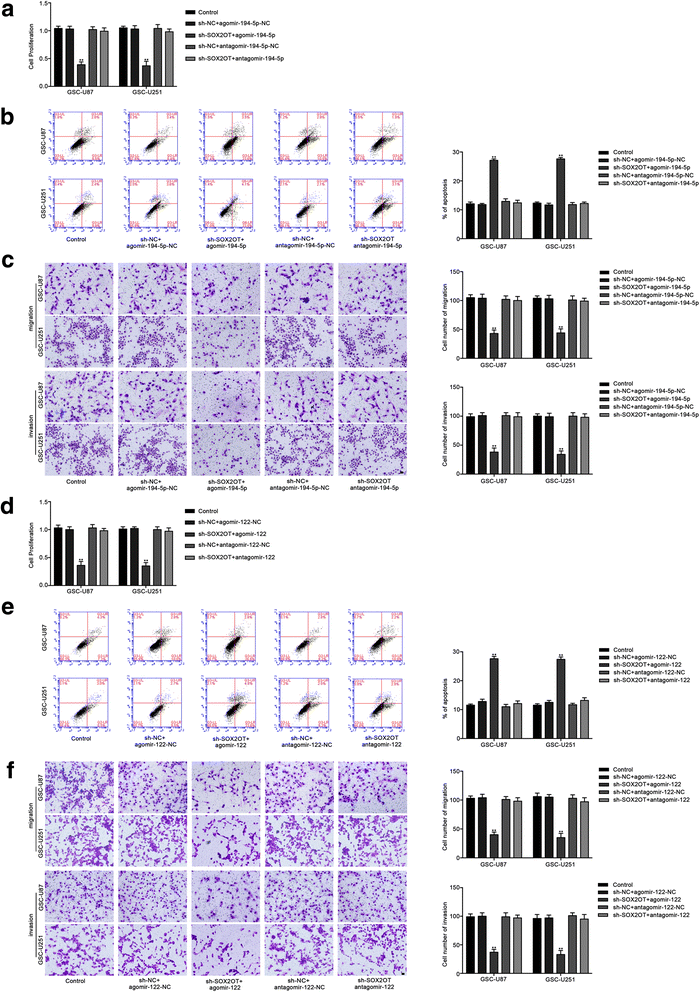
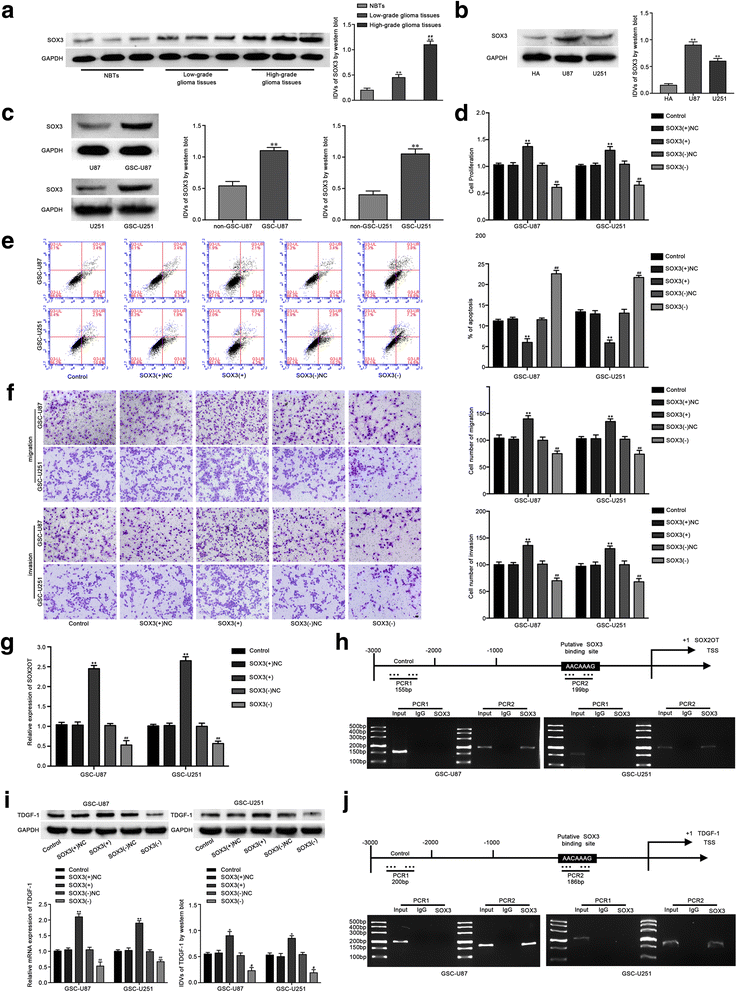
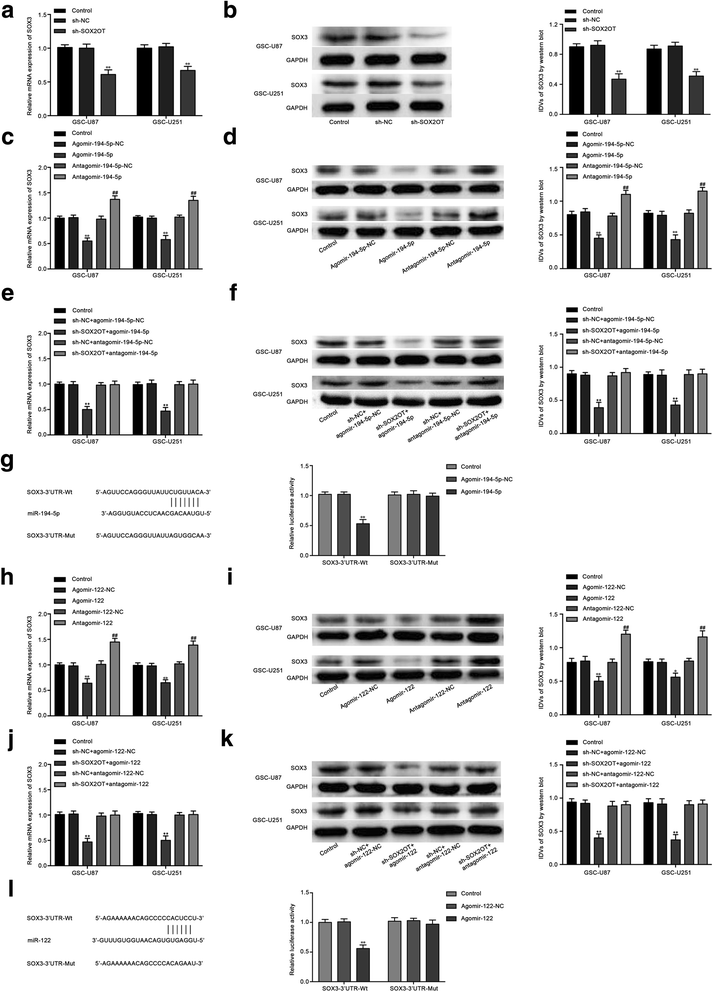
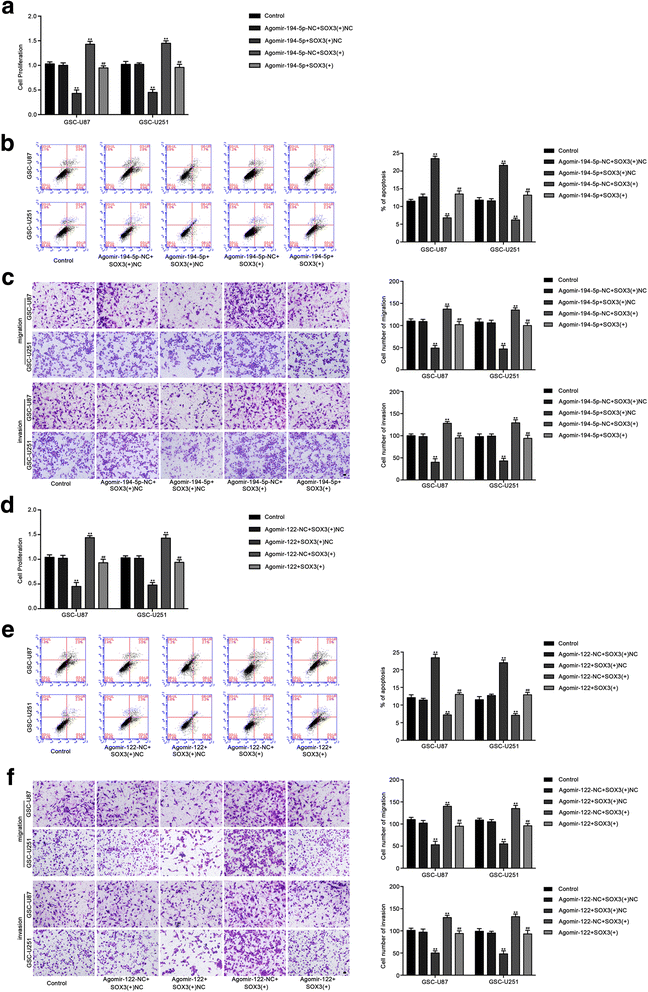
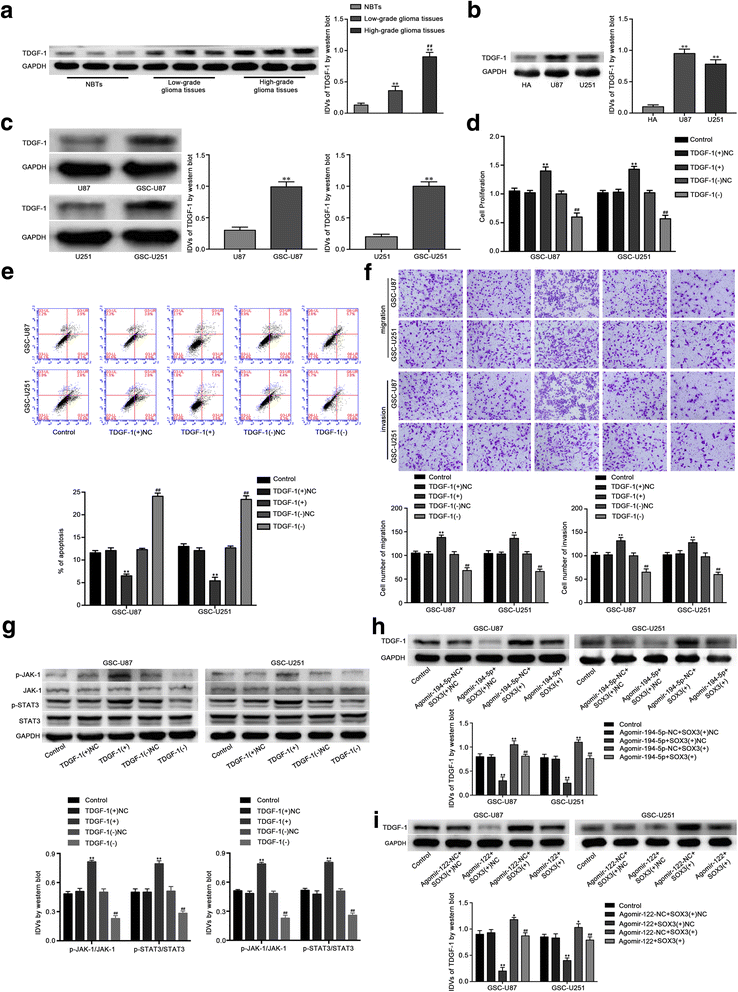
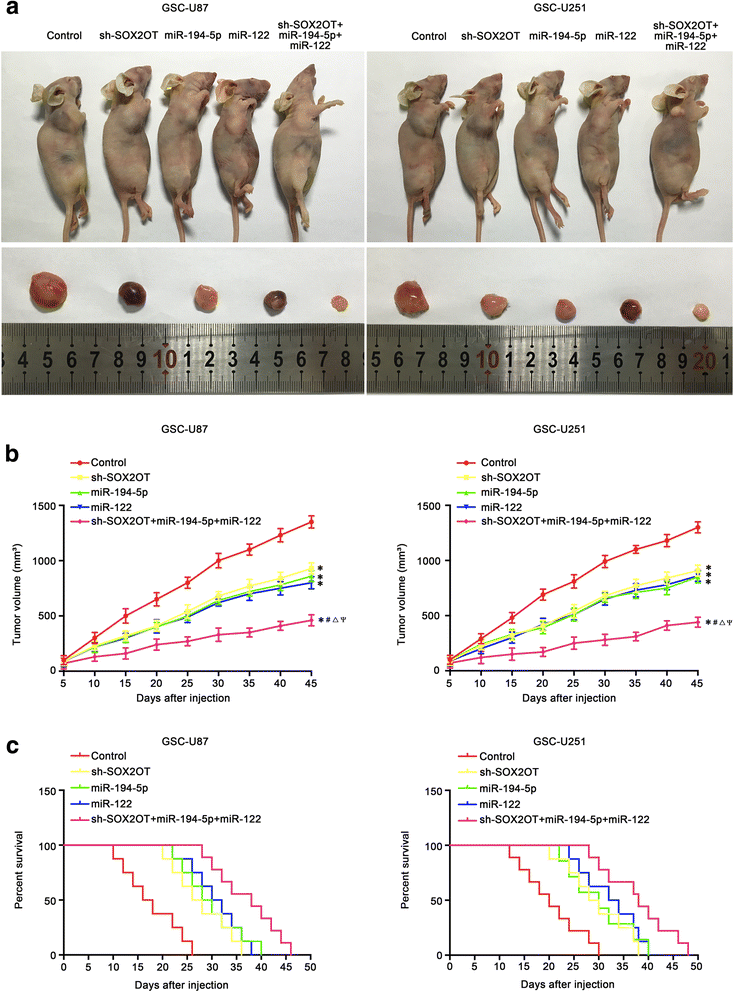
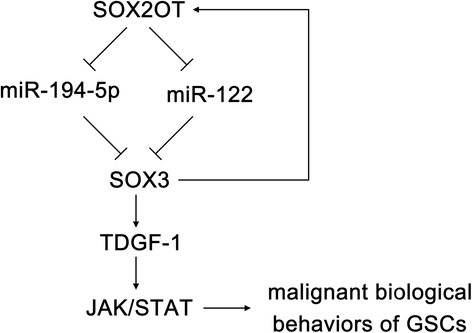
Results: Real-time PCR demonstrated that SOX2OT expression was up-regulated in glioma tissues and GSCs. Knockdown of SOX2OT inhibited the proliferation, migration and invasion of GSCs, and promoted GSCs apoptosis. MiR-194-5p and miR-122 were down-regulated in human glioma tissues and GSCs, and miR-194-5p and miR-122 respectively exerted tumor-suppressive functions by inhibiting the proliferation, migration and invasion of GSCs, while promoting GSCs apoptosis. Knockdown of SOX2OT significantly increased the expression of miR-194-5p and miR-122 in GSCs. Dual-luciferase reporter assay revealed that SOX2OT bound to both miR-194-5p and miR-122. SOX3 and TDGF-1 were up-regulated in human glioma tissues and GSCs. Knockdown of SOX3 inhibited the proliferation, migration and invasion of GSCs, promoted GSCs apoptosis, and decreased TDGF-1 mRNA and protein expression through direct binding to the TDGF-1 promoter. Over-expression of miR-194-5p and miR-122 decreased the mRNA and protein expression of SOX3 by targeting its 3'UTR. Knockdown of TDGF-1 inhibited the proliferation, migration and invasion of GSCs, promoted GSCs apoptosis, and inhibited the JAK/STAT signaling pathway. Furthermore, SOX3 knockdown also inhibited the SOX2OT expression through direct binding to the SOX2OT promoter and formed a positive feedback loop.
Conclusion: This study is the first to demonstrate that the SOX2OT-miR-194-5p/miR-122-SOX3-TDGF-1 pathway forms a positive feedback loop and regulates the biological behaviors of GSCs, and these findings might provide a novel strategy for glioma treatment.
Keywords: Glioma; SOX2OT; SOX3; TDGF-1; miR-122; miR-194-5p.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

