Yeast interfering RNA larvicides targeting neural genes induce high rates of Anopheles larval mortality
- PMID: 29132374
- PMCID: PMC5683233
- DOI: 10.1186/s12936-017-2112-5
Yeast interfering RNA larvicides targeting neural genes induce high rates of Anopheles larval mortality
Abstract
Background: Although larviciding can reduce the number of outdoor biting malaria vector mosquitoes, which may help to prevent residual malaria transmission, the current larvicide repertoire is faced with great challenges to sustainability. The identification of new effective, economical, and biorational larvicides could facilitate maintenance and expansion of the practice of larviciding in integrated malaria vector mosquito control programmes. Interfering RNA molecules represent a novel class of larvicides with untapped potential for sustainable mosquito control. This investigation tested the hypothesis that short interfering RNA molecules can be used as mosquito larvicides.
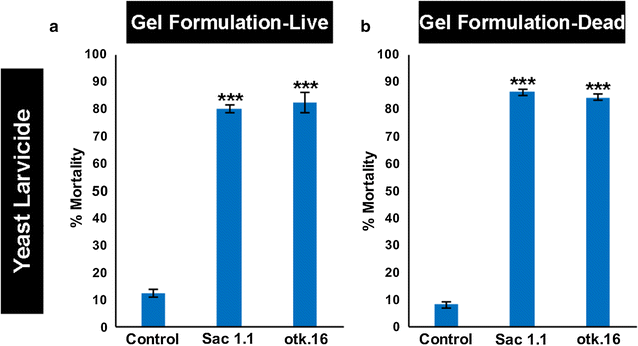
Results: A small interfering RNA (siRNA) screen for larval lethal genes identified siRNAs corresponding to the Anopheles gambiae suppressor of actin (Sac1), leukocyte receptor complex member (lrc), and offtrack (otk) genes. Saccharomyces cerevisiae (baker's yeast) was engineered to produce short hairpin RNAs (shRNAs) for silencing of these genes. Feeding larvae with the engineered yeasts resulted in silenced target gene expression, a severe loss of neural synapses in the larval brain, and high levels of larval mortality. The larvicidal activities of yeast interfering RNA larvicides were retained following heat inactivation and drying of the yeast into user-friendly tablet formulations that induced up to 100% larval mortality in laboratory trials.
Conclusions: Ready-to-use dried inactivated yeast interfering RNA larvicide tablets may someday be an effective and inexpensive addition to malaria mosquito control programmes and a valuable, biorational tool for addressing residual malaria transmission.
Keywords: Brain; Larvae; Larviciding; Malaria; Mosquito; Pesticide; RNAi; Saccharomyces cerevisiae; Synapse; Vector.
Figures
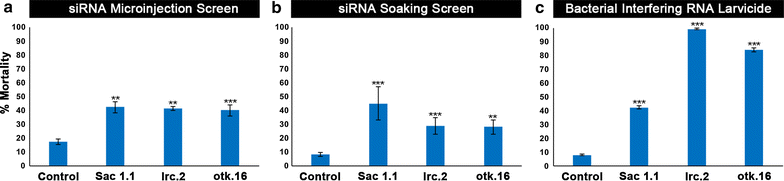



References
-
- WHO. World malaria report 2016. Geneva: World Health Organization; 2016.
-
- WHO. Larval source management: a supplementary measure for malaria vector control: an operational manual. Geneva: World Health Organization; 2013.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

