EZH2 suppression in glioblastoma shifts microglia toward M1 phenotype in tumor microenvironment
- PMID: 29132376
- PMCID: PMC5684749
- DOI: 10.1186/s12974-017-0993-4
EZH2 suppression in glioblastoma shifts microglia toward M1 phenotype in tumor microenvironment
Abstract
Background: Glioblastoma multiforme (GBM) induces tumor immunosuppression through interacting with tumor-infiltrating microglia or macrophages (TAMs) with an unclear pathogenesis. Enhancer of zeste homolog 2 (EZH2) is abundant in GBM samples and cell lines and is involved in GBM proliferation, cell cycle, and invasion, whereas its association with innate immune response is not yet reported. Herein, the aim of this study was to investigate the role of EZH2 in GBM immune.
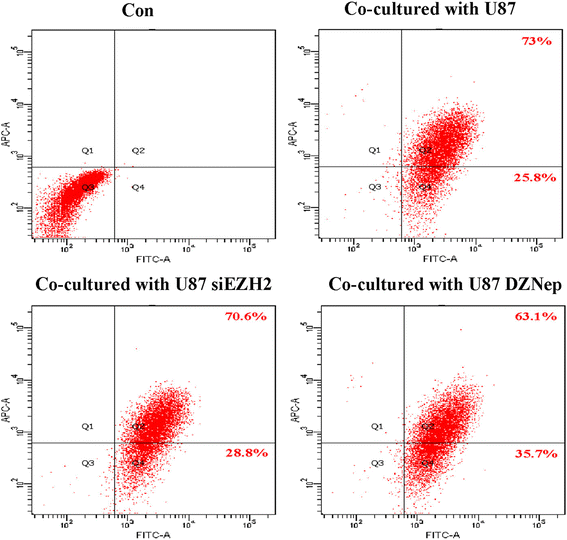
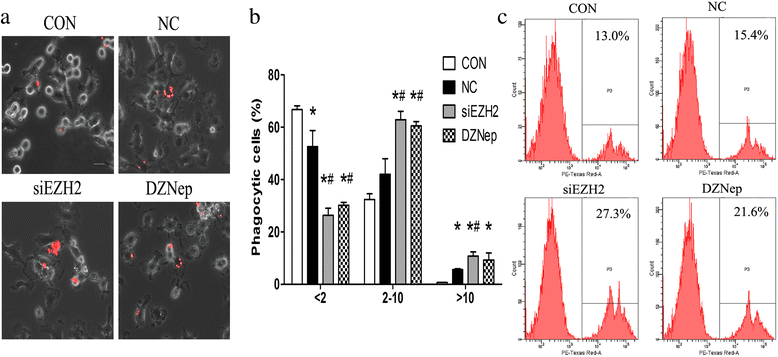
Methods: Co-culturing models of human/murine GBM cells with PBMC-derived macrophages/primary microglia were employed. EZH2 mRNAs and function were suppressed by siEZH2 and DZNep. Real-time PCR and flow cytometry were used to determine levels of microglia/macrophages markers. The fluorescence-labeled latex beads and flow cytometry were utilized to evaluate phagocytic abilities of microglia. CCK8 assay was performed to assess microglia proliferation.
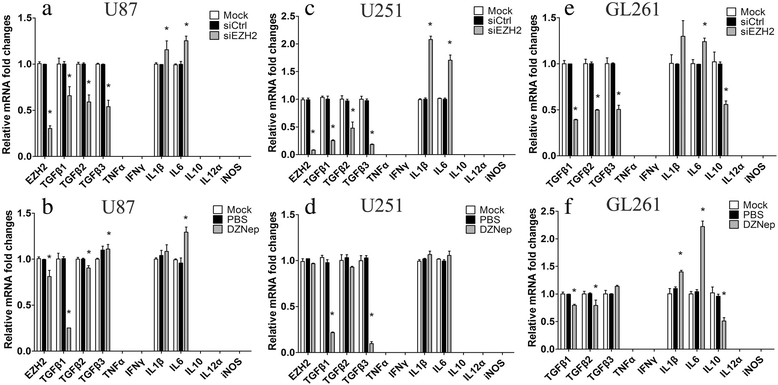
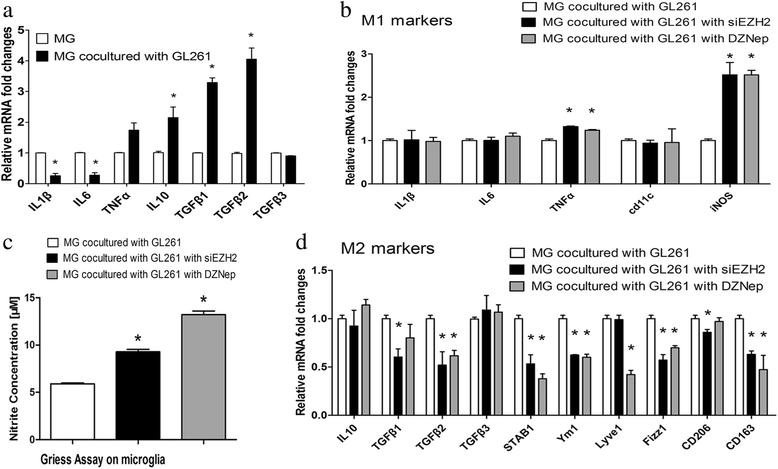
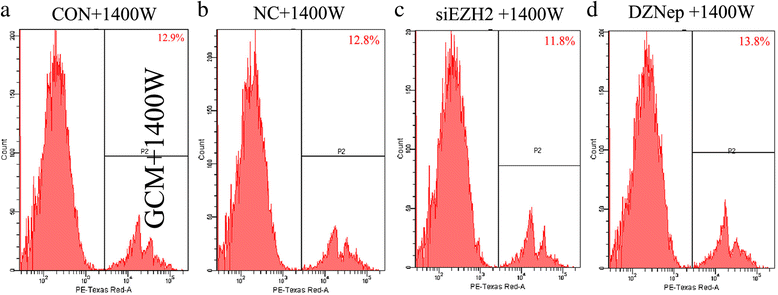
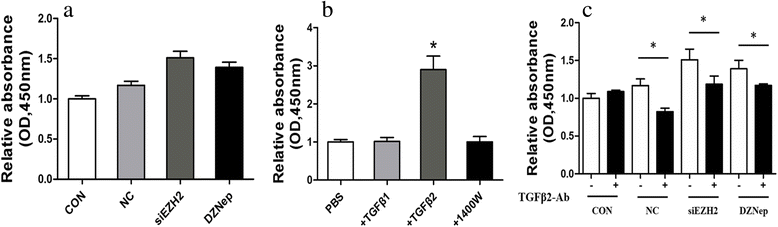
Results: EZH2 inhibition led to significant reduction of TGFβ1-3 and IL10 and elevation of IL1β and IL6 in human and murine GBM cells. More importantly, EZH2 suppression in GBM cells resulted in significant increase of M1 markers (TNFα and iNOS) and decrease of a pool of M2 markers in murine microglia. The proportion of CD206+ cells was decreased in PBMC-derived macrophages as co-incubated with EZH2-inhibited GBM cells. Functional researches showed that phagocytic capacities of microglia were significantly ameliorated after EZH2 inhibition in co-culturing GBM cells and microglia proliferation was declined after addition of TGFβ2 antibodies to co-incubated GBM cells with EZH2 inhibition. Besides, we found that EZH2 suppression in GBM cells enhanced co-culturing microglia engulfment through activation of iNOS.
Conclusions: Our data demonstrates that EZH2 participates in GBM-induced immune deficient and EZH2 suppression in GBM can remodel microglia immune functions, which is beneficial for understanding GBM pathogenesis and suggests potential targets for therapeutic approaches.
Keywords: Enhancer of zeste homolog 2 (EZH2); Glioblastoma; Microglia; Polarization.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the ethics committee at Sun Yat-sen Memorial Hospital, Sun Yat-sen University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

