Interferon-γ-inducible protein 16 (IFI16) is required for the maintenance of Epstein-Barr virus latency
- PMID: 29132393
- PMCID: PMC5683537
- DOI: 10.1186/s12985-017-0891-5
Interferon-γ-inducible protein 16 (IFI16) is required for the maintenance of Epstein-Barr virus latency
Abstract
Background: Epstein-Barr virus (EBV) exhibits both lytic and latent (Lat. I, II, and III) phases in an infected individual. It's during the latent phase of EBV that all EBV-associated cancers, including Burkitt's lymphoma, nasopharyngeal carcinoma and lymphoproliferative disease arise. Interferon-γ-inducible protein 16 (IFI16) is a well-established innate immune sensor and viral transcriptional regulator involved in response to invading DNA viruses. During latency, IFI16 remains in the nucleus, in part bound to the EBV genome; however, neither its role in EBV lytic cycle or latency has been established.
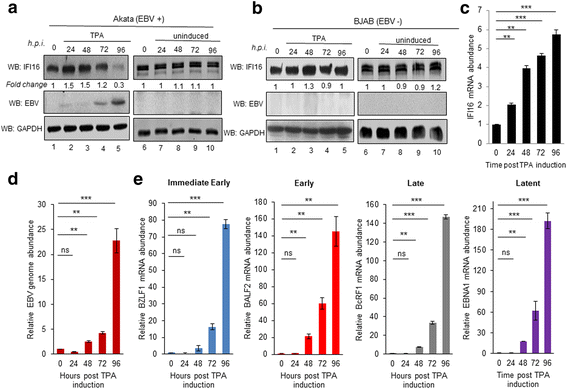
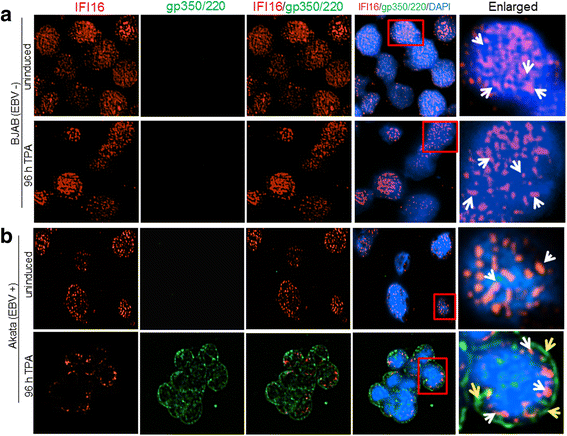
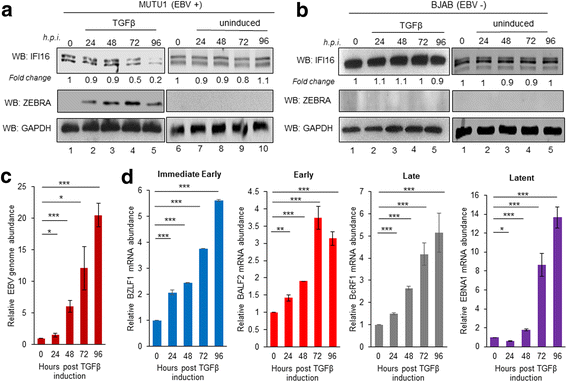
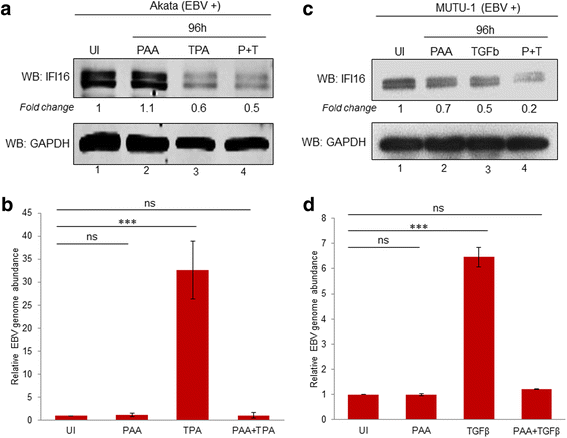
Methods: Short interfering RNA against IFI16 and IFI16 overexpression were used to identify the role of IFI16 in the maintenance of EBV latency I. We also studied how induction of the lytic cycle affected IFI16 using the EBV positive, latently infected Akata or MUTU-1 cell lines. Akata cells were induced with TPA and MUTU-1 cells with TGF-β up to 96 h and changes in IFI16 protein were analyzed by Western blotting and immunofluorescence microscopy. To assess the mechanism of IFI16 decrease, EBV DNA replication and late lytic transcripts were blocked using the viral DNA polymerase inhibitor phosphonoacetic acid.
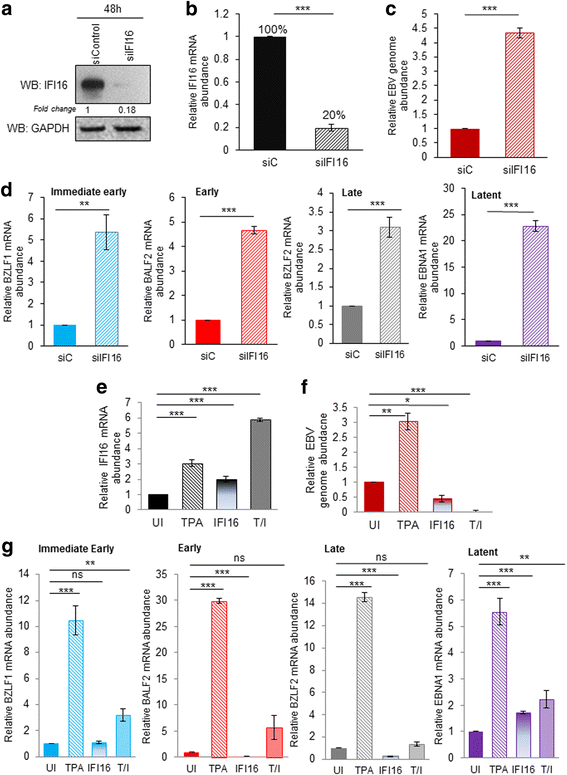
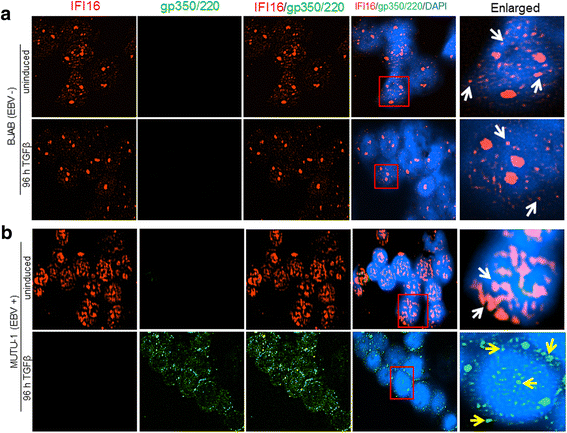
Results: Knockdown of IFI16 mRNA by siRNA resulted in enhanced levels of EBV lytic gene expression from all temporal gene classes, as well as an increase in the total EBV genome abundance, whereas overexpression of exogenous IFI16 reversed these effects. Furthermore, 96 h after induction of the lytic cycle with either TPA (Akata) or TGF-β (MUTU-1), IFI16 protein levels decreased up to 80% as compared to the EBV-negative cell line BJAB. Reduction in IFI16 was observed in cells expressing EBV lytic envelope glycoprotein. The decreased levels of IFI16 protein do not appear to be dependent on late lytic transcripts of EBV but suggest involvement of the immediate early, early, or a combination of both gene classes.
Conclusions: Reduction of IFI16 protein levels following lytic cycle induction, as well as reactivation from latency after IFI16 mRNA knockdown suggests that IFI16 is crucial for the maintenance of EBV latency. More importantly, these results identify IFI16 as a unique host factor protein involved in the EBV lifecycle, making it a potential therapeutic target to combat EBV-related malignancies.
Keywords: Epstein-Barr virus (EBV); Herpesvirus; Interferon-γ-inducible protein 16 (IFI16); Latency; Lytic cycle.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
We have obtained written consent from all participants to publish their data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Nuclear Innate Immune DNA Sensor IFI16 Is Degraded during Lytic Reactivation of Kaposi's Sarcoma-Associated Herpesvirus (KSHV): Role of IFI16 in Maintenance of KSHV Latency.J Virol. 2016 Sep 12;90(19):8822-41. doi: 10.1128/JVI.01003-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27466416 Free PMC article.
-
IFI16 Partners with KAP1 to Maintain Epstein-Barr Virus Latency.J Virol. 2022 Sep 14;96(17):e0102822. doi: 10.1128/jvi.01028-22. Epub 2022 Aug 15. J Virol. 2022. PMID: 35969079 Free PMC article.
-
IRF4 promotes Epstein-Barr virus activation in Burkitt's lymphoma cells.J Gen Virol. 2019 May;100(5):851-862. doi: 10.1099/jgv.0.001249. Epub 2019 Mar 25. J Gen Virol. 2019. PMID: 30907723
-
Epstein-Barr Virus Hijacks DNA Damage Response Transducers to Orchestrate Its Life Cycle.Viruses. 2017 Nov 16;9(11):341. doi: 10.3390/v9110341. Viruses. 2017. PMID: 29144413 Free PMC article. Review.
-
Reactivation of Epstein-Barr virus from latency.Rev Med Virol. 2005 May-Jun;15(3):149-56. doi: 10.1002/rmv.456. Rev Med Virol. 2005. PMID: 15546128 Review.
Cited by
-
Barrier-to-autointegration factor 1 promotes gammaherpesvirus reactivation from latency.Nat Commun. 2023 Feb 6;14(1):434. doi: 10.1038/s41467-023-35898-2. Nat Commun. 2023. PMID: 36746947 Free PMC article.
-
Epstein-Barr Virus and Innate Immunity: Friends or Foes?Microorganisms. 2019 Jun 24;7(6):183. doi: 10.3390/microorganisms7060183. Microorganisms. 2019. PMID: 31238570 Free PMC article. Review.
-
Epigenetic Restriction Factors (eRFs) in Virus Infection.Viruses. 2024 Jan 25;16(2):183. doi: 10.3390/v16020183. Viruses. 2024. PMID: 38399958 Free PMC article. Review.
-
Epstein-Barr Virus Synergizes with BRD7 to Conquer c-Myc-Mediated Viral Latency Maintenance via Chromatin Remodeling.Microbiol Spectr. 2023 Feb 2;11(2):e0123722. doi: 10.1128/spectrum.01237-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36728436 Free PMC article.
-
Rasmussen's encephalitis is characterized by relatively lower production of IFN-β and activated cytotoxic T cell upon herpes viruses infection.J Neuroinflammation. 2022 Mar 26;19(1):70. doi: 10.1186/s12974-022-02379-0. J Neuroinflammation. 2022. PMID: 35337341 Free PMC article.
References
-
- Anagnostopoulos I, Hummel M, Kreschel C, Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995;85:744–750. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

