Comparison of the efficacy and safety of FP-1201-lyo (intravenously administered recombinant human interferon beta-1a) and placebo in the treatment of patients with moderate or severe acute respiratory distress syndrome: study protocol for a randomized controlled trial
- PMID: 29132404
- PMCID: PMC5683224
- DOI: 10.1186/s13063-017-2234-7
Comparison of the efficacy and safety of FP-1201-lyo (intravenously administered recombinant human interferon beta-1a) and placebo in the treatment of patients with moderate or severe acute respiratory distress syndrome: study protocol for a randomized controlled trial
Abstract
Background: Acute respiratory distress syndrome (ARDS) results in vascular leakage, inflammation and respiratory failure. There are currently no approved pharmacological treatments for ARDS and standard of care involves treatment of the underlying cause, and supportive care. The vascular leakage may be related to reduced concentrations of local adenosine, which is involved in maintaining endothelial barrier function. Interferon (IFN) beta-1a up-regulates the cell surface ecto-5'-nucleotidase cluster of differentiation 73 (CD73), which increases adenosine levels, and IFN beta-1 may, therefore, be a potential treatment for ARDS. In a phase I/II, open-label study in 37 patients with acute lung injury (ALI)/ARDS, recombinant human IFN beta-1a was well tolerated and mortality rates were significantly lower in treated than in control patients.
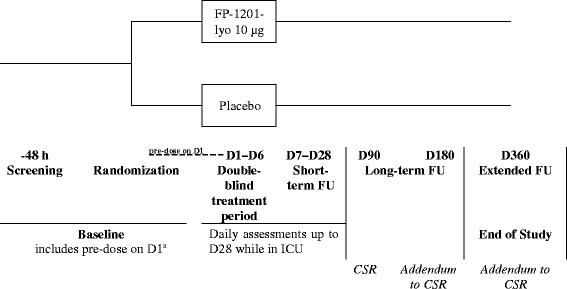
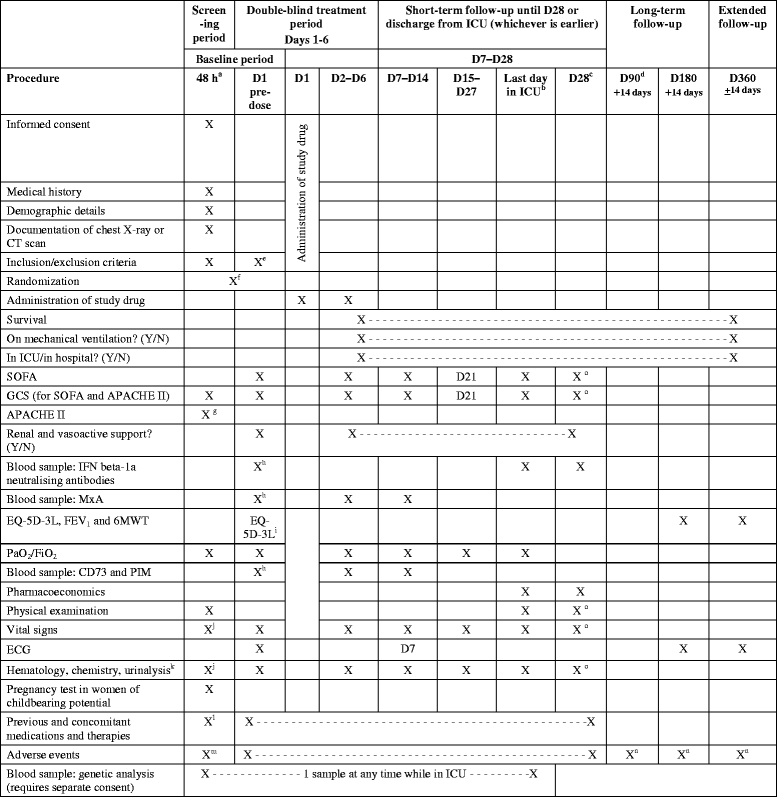
Methods/design: In this phase III, double-blind, randomized, parallel-group trial, the efficacy and safety of recombinant human IFN beta-1a (FP-1201-lyo) will be compared with placebo in adult patients with ARDS. Patients will be randomly assigned to receive 10 μg FP-1201-lyo or placebo administered intravenously once daily for 6 days and will be monitored for 28 days or until discharged from the intensive care unit. Follow-up visits will then take place at days 90, 180 and 360. The primary endpoint is a composite endpoint including any cause of death at 28 days and days free of mechanical ventilation within 28 days among survivors. Secondary endpoints include: all-cause mortality at 28, 90, 180 and 360 days; organ failure-free days; length of hospital stay; pharmacodynamic assessment including measurement of myxovirus resistance protein A concentrations; and measures of quality of life, respiratory and neurological function at 180 and 360 days. The estimated sample size to demonstrate a reduction in the primary outcome between groups from 30% to 15% is 300 patients, and the study will be conducted in 70-80 centers in nine countries across Europe.
Discussion: There are no effective specific treatments for patients with ARDS and mortality rates remain high. The results from this study will provide evidence regarding the efficacy of a potential new therapeutic agent, FP-1201-lyo, in improving the clinical course and outcome for patients with moderate/severe ARDS.
Trial registration: European Union Clinical Trials Register, no: 2014-005260-15 . Registered on 15 July 2017.
Keywords: ARDS; CD73; Interferon; Vascular leakage.
Conflict of interest statement
Ethics approval and consent to participate
The Local Ethics Committee at each site will approve the study protocol (approvals already in place shown in Additional file 2). Any modifications to the protocol will be immediately communicated to all responsible authorities. All patients, or their legal representative, must give written informed consent before study participation (model form, Additional file 4). The patient or their representative will also be asked to give separate consent for a genetic sample to be taken. Consent for genetic sampling is not a prerequisite for study participation.
Consent for publication
Results obtained in this trial will be published in an international journal and may be presented at international scientific meetings. This will be included in the patient consent form. There are no plans for data sharing. Results suggested for presentation or publication will be circulated to the Steering Committee members representing each participating country and the sponsor, Faron Pharmaceuticals Ltd. Authorship of any publications will be granted based on standard authorship criteria and will be decided based on the contributions to the design, conduct, interpretation, and reporting of the INTEREST trial. Disputes regarding authorship will be settled by the sponsor. Professional medical writers may be contracted to improve clarity and structure in trial-related reports.
Competing interests
Mikael Maksimow, Markku Jalkanen and Ilse Piippo are employed by Faron Pharmaceuticals and hold Faron shares and/or options for shares. The other authors are members of the INTEREST trial Steering Committee and have received expenses only for participation in required study meetings.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

