AC-93253 iodide, a novel Src inhibitor, suppresses NSCLC progression by modulating multiple Src-related signaling pathways
- PMID: 29132432
- PMCID: PMC5683468
- DOI: 10.1186/s13045-017-0539-3
AC-93253 iodide, a novel Src inhibitor, suppresses NSCLC progression by modulating multiple Src-related signaling pathways
Abstract
Background: The tyrosine kinase Src is involved in the progression of many cancers. Moreover, inhibiting Src activity has been shown to obstruct several signaling pathways regulated by the EGFR. Thus, Src is a valuable target molecule in drug development. The purpose of this study was to identify compounds that directly or indirectly modulate Src to suppress lung cancer cell growth and motility and to investigate the molecular mechanisms underlying the effects of these compounds.
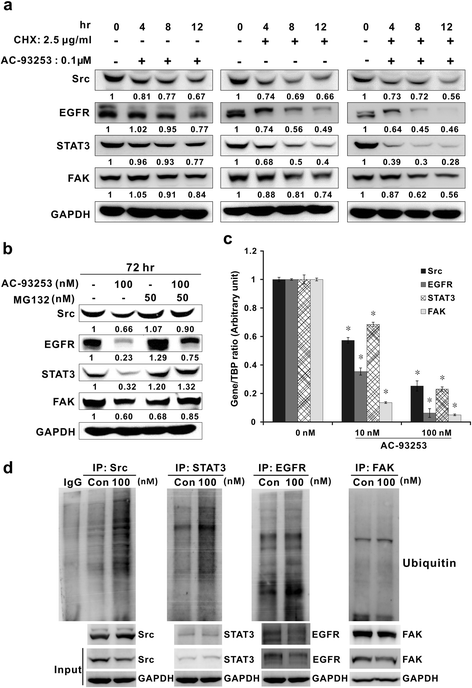
Methods: Human non-small cell lung cancer (NSCLC) cell lines (PC9, PC9/gef, A549, and H1975) with different EGFR statuses were tested by cytotoxicity and proliferation assays after AC-93253 iodide treatment. Src and Src-related protein expression in AC-93253 iodide-treated PC9, PC9/gef, and A549 cells were assessed by western blotting. The effects of AC-93253 iodide on cancer cell colony formation, invasion, and migration were assessed in PC9 and PC9/gef cells. The synergistic effects of gefitinib and AC-93253 iodide were evaluated by combination index (CI)-isobologram analysis in gefitinib-resistant cell lines. The efficacy of AC-93253 iodide in vivo was determined using nude mice treated with either the compound or the vehicle.
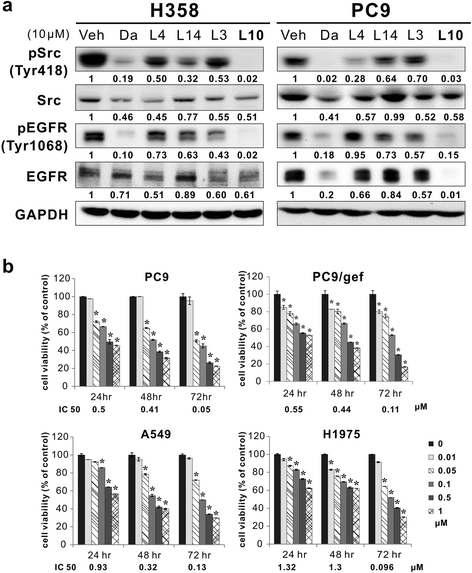
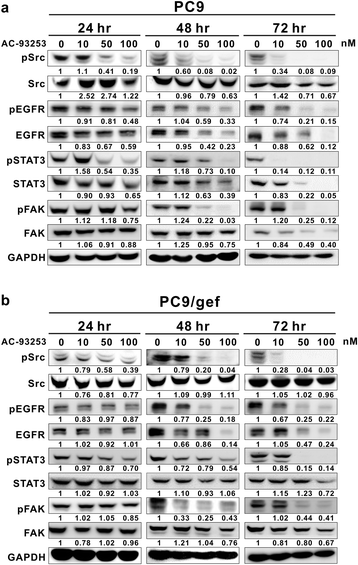
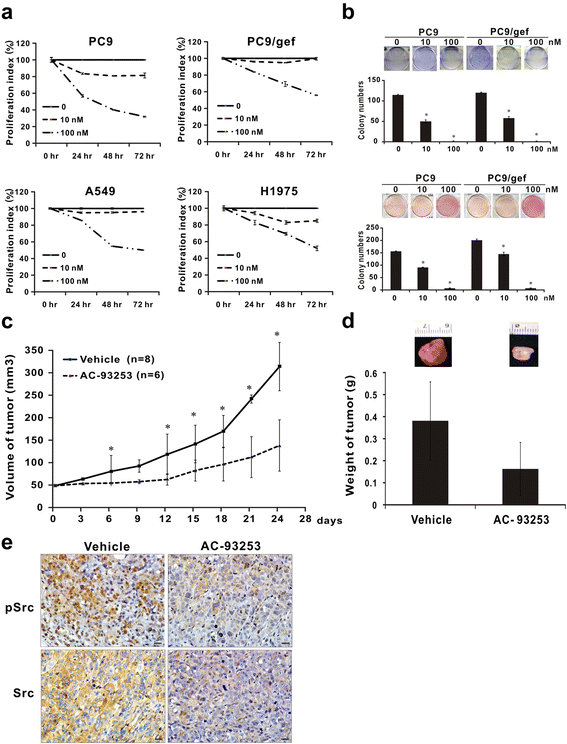
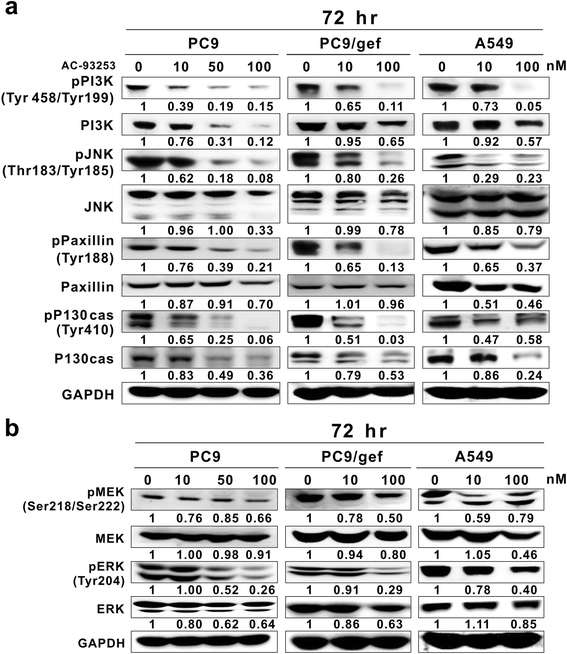
Results: Among the compounds, AC-93253 iodide exhibited the most potent dose-independent inhibitory effects on the activity of Src as well as on that of the Src-related proteins EGFR, STAT3, and FAK. Furthermore, AC-93253 iodide significantly suppressed cancer cell proliferation, colony formation, invasion, and migration in vitro and tumor growth in vivo. AC-93253 iodide sensitized tumor cells to gefitinib treatment regardless of whether the cells were gefitinib-sensitive (PC9) or resistant (H1975 and PC9/gef), indicating that it may exert synergistic effects when used in combination with established therapeutic agents. Our findings also suggested that the inhibitory effects of AC-93253 iodide on lung cancer progression may be attributable to its ability to modulate multiple proteins, including Src, PI3K, JNK, Paxillin, p130cas, MEK, ERK, and EGFR.
Conclusions: Our data suggest that AC-93253 iodide inhibits NSCLC cell growth and motility by regulating multiple Src-related pathways. Our findings may facilitate the development of therapeutic strategies and anti-tumor drugs that may be useful for treating lung cancer in the future.
Keywords: AC-93253 iodide; EGFR; Gefitinib; NSCLC; Src.
Conflict of interest statement
Ethics approval
All animal studies were performed with the approval from the Institutional Animal Care and Use Committee (IACUC) of National Chung Hsing University (IACUC Approval No. 102-125).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Sequist LV, Heist RS, Shaw AT, Fidias P, Rosovsky R, Temel JS, Lennes IT, Digumarthy S, Waltman BA, Bast E, Tammireddy S, Morrissey L, Muzikansky A, Goldberg SB, Gainor J, Channick CL, Wain JC, Gaissert H, Donahue DM, Muniappan A, Wright C, Willers H, Mathisen DJ, Choi NC, Baselga J, Lynch TJ, Ellisen LW, Mino-Kenudson M, Lanuti M, Borger DR, Iafrate AJ, Engelman JA, Dias-Santagata D. Implementing multiplexed genotyping of non-small-cell lung cancers into routine clinical practice. Ann Oncol. 2011;22(12):2616–2624. doi: 10.1093/annonc/mdr489. - DOI - PMC - PubMed
-
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–1075. doi: 10.1038/nature07423. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

