Early screening for Chlamydia trachomatis in young women for primary prevention of pelvic inflammatory disease (i-Predict): study protocol for a randomised controlled trial
- PMID: 29132441
- PMCID: PMC5683219
- DOI: 10.1186/s13063-017-2211-1
Early screening for Chlamydia trachomatis in young women for primary prevention of pelvic inflammatory disease (i-Predict): study protocol for a randomised controlled trial
Abstract
Background: Genital infection with Chlamydia trachomatis (Ct) is the most common bacterial sexually transmitted infection, especially among young women. Mostly asymptomatic, it can lead, if untreated, to pelvic inflammatory disease (PID), tubal factor infertility and ectopic pregnancy. Recent data suggest that Ct infections are not controlled in France and in Europe. The effectiveness of a systematic strategy for Ct screening in under-25 women remains controversial. The main objective of the i-Predict trial (Prevention of Diseases Induced by Chlamydia trachomatis) is to determine whether early screening and treatment of 18- to-24-year-old women for genital Ct infection reduces the incidence of PID over 24 months.
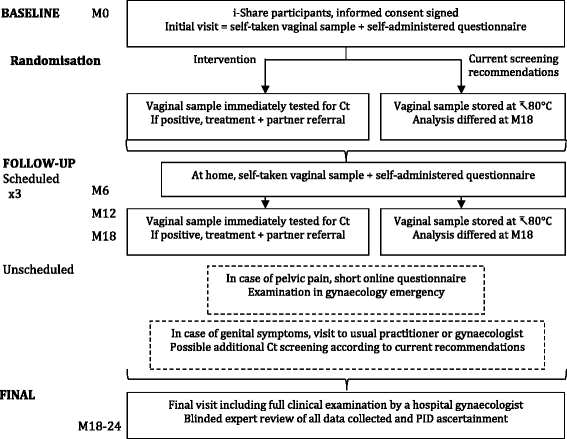
Methods/design: This is a randomised prevention trial including 4000 eighteen- to twenty-four-year-old sexually active female students enrolled at five universities. The participants will provide a self-collected vaginal swab sample and fill in an electronic questionnaire at baseline and at 6, 12 and 18 months after recruitment. Vaginal swabs in the intervention arm will be analysed immediately for Ct positivity, and participants will be referred for treatment if they have a positive test result. Vaginal swabs from the control arm will be analysed at the end of the study. All visits to general practitioners, gynaecologists or gynaecology emergency departments for pelvic pain or other gynaecological symptoms will be recorded to evaluate the incidence of PID, and all participants will attend a final visit in a hospital gynaecology department. The primary endpoint measure will be the incidence of PID over 24 months. The outcome status (confirmed, probable or no PID) will be assessed by two independent experts blinded to group assignment and Ct status.
Discussion: This trial is expected to largely contribute to the development of recommendations for Ct screening in young women in France to prevent PID and related complications. It is part of a comprehensive approach to gathering data to facilitate decision-making regarding optimal strategies for Ct infection control. The control group of this randomised trial, following current recommendations, will allow better documentation of the natural history of Ct infection, a prerequisite to evaluating the impact of Ct screening. Characterisation of host immunogenetics will also allow identification of women at risk for complications.
Trial registration: ClinicalTrials.gov, NCT02904811 . Registered on September 14, 2016. World Health Organisation International Clinical Trials Registry, NCT02904811. AOM, 15-0063 and P150950. Registered on September 26, 2016. A completed Standard Protocol Items : Recommendations for International Trials (SPIRIT) Checklist is available in additional file 1.
Keywords: Chlamydia trachomatis; Clearance; Immunogenetics; Infection; Natural course of infection; Pelvic inflammatory disease; Prevention; Reinfection; Screening; Students.
Conflict of interest statement
Ethics approval and consent to participate
The i-Predict trial obtained favourable opinions from the Comité de Protection des Personnes (CPP) on June 2, 2016 (CPP number 2016/16, reference number P150950); from the Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé (CCTIRS) on May 19, 2016 (reference number 16-372); and from the Commission National Informatique et Libertés (CNIL) on August 1, 2016 (reference number MMS/ABD/AR168237, decision number DR-2016-350). This trial was authorised by the Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) on May 30, 2016 (reference IDRCB 2016-A00491-50). In case the protocol needs to be modified, the investigator-coordinator is expected to submit a request to the CPP and send an information note to all the investigators. All participants in the study will sign an informed consent form prior to participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10(12):e0143304. doi: 10.1371/journal.pone.0143304. - DOI - PMC - PubMed
-
- de Barbeyrac B, Kersaudy-Rahib D, de Diego S, Le Roy C, Bébéar C, Lydié N. Internet testing for Chlamydia trachomatis in France in 2012 [abstract P3.025] Sex Transm Infect. 2013;89(Suppl 1):A155–6. doi: 10.1136/sextrans-2013-051184.0485. - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical