Taming the Beast: Control of APC/CCdc20-Dependent Destruction
- PMID: 29133301
- PMCID: PMC6374126
- DOI: 10.1101/sqb.2017.82.033712
Taming the Beast: Control of APC/CCdc20-Dependent Destruction
Abstract
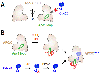
The anaphase-promoting complex/cyclosome (APC/C) is a large multisubunit ubiquitin ligase that triggers the metaphase-to-anaphase transition in the cell cycle by targeting the substrates cyclin B and securin for destruction. APC/C activity toward these two key substrates requires the coactivator Cdc20. To ensure that cells enter mitosis and partition their duplicated genome with high accuracy, APC/CCdc20 activity must be tightly controlled. Here, we discuss the mechanisms that regulate APC/CCdc20 activity both before and during mitosis. We focus our discussion primarily on the chromosomal pathways that both accelerate and delay APC/C activation by targeting Cdc20 to opposing fates. The findings discussed provide an overview of how cells control the activation of this major cell cycle regulator to ensure both accurate and timely cell division.
© 2017 Lara-Gonzalez et al; Published by Cold Spring Harbor Laboratory Press.
Figures

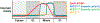
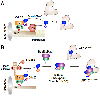
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
