Study protocol for THINK: a multinational open-label phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types
- PMID: 29133316
- PMCID: PMC5695348
- DOI: 10.1136/bmjopen-2017-017075
Study protocol for THINK: a multinational open-label phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types
Abstract
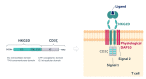
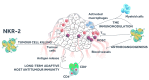
Introduction: NKR-2 are autologous T cells genetically modified to express a chimeric antigen receptor (CAR) comprising a fusion of the natural killer group 2D (NKG2D) receptor with the CD3ζ signalling domain, which associates with the adaptor molecule DNAX-activating protein of 10 kDa (DAP10) to provide co-stimulatory signal upon ligand binding. NKG2D binds eight different ligands expressed on the cell surface of many tumour cells and which are normally absent on non-neoplastic cells. In preclinical studies, NKR-2 demonstrated long-term antitumour activity towards a breadth of tumour indications, with maximum efficacy observed after multiple NKR-2 administrations. Importantly, NKR-2 targeted tumour cells and tumour neovasculature and the local tumour immunosuppressive microenvironment and this mechanism of action of NKR-2 was established in the absence of preconditioning.
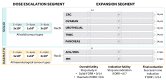
Methods and analysis: This open-label phase I study will assess the safety and clinical activity of NKR-2 treatment administered three times, with a 2-week interval between each administration in different tumour types. The study will contain two consecutive segments: a dose escalation phase followed by an expansion phase. The dose escalation study involves two arms, one in solid tumours (five specific indications) and one in haematological tumours (two specific indications) and will include three dose levels in each arm: 3×108, 1×109 and 3×109 NKR-2 per injection. On the identification of the recommended dose in the first segment, based on dose-limiting toxicity occurrences, the study will expand to seven different cohorts examining the seven different tumour types separately. Clinical responses will be determined according to standard Response Evaluation Criteria In Solid Tumors (RECIST) criteria for solid tumours or international working group response criteria in haematological tumours.
Ethics approval and dissemination: Ethical approval has been obtained at all sites. Written informed consent will be taken from all participants. The results of this study will be disseminated through presentation at international scientific conferences and reported in peer-reviewed scientific journals.
Trial registration number: NCT03018405, EudraCT 2016-003312-12; Pre-result.
Keywords: car-t; chimeric antigen receptor; multiple injections; nkg2d; nkr-2; solid tumours.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: CL, BV, DEG and FFL are employed by Celyad SA. JBB and PA declare no disclosures. J-PHM has advisory roles with Merck Sharp and Dohme (uncompensated), Innate Pharma, Debio, Boehringer-Ingelheim and Nanobiotix. The THINK clinical trial is sponsored by Celyad SA.
Figures
Similar articles
-
CYAD-01, an autologous NKG2D-based CAR T-cell therapy, in relapsed or refractory acute myeloid leukaemia and myelodysplastic syndromes or multiple myeloma (THINK): haematological cohorts of the dose escalation segment of a phase 1 trial.Lancet Haematol. 2023 Mar;10(3):e191-e202. doi: 10.1016/S2352-3026(22)00378-7. Epub 2023 Feb 7. Lancet Haematol. 2023. PMID: 36764323 Clinical Trial.
-
Manufacturing development and clinical production of NKG2D chimeric antigen receptor-expressing T cells for autologous adoptive cell therapy.Cytotherapy. 2018 Jul;20(7):952-963. doi: 10.1016/j.jcyt.2018.05.001. Epub 2018 Jun 29. Cytotherapy. 2018. PMID: 30180944 Free PMC article.
-
Solid tumor immunotherapy using NKG2D-based adaptor CAR T cells.Cell Rep Med. 2024 Nov 19;5(11):101827. doi: 10.1016/j.xcrm.2024.101827. Cell Rep Med. 2024. PMID: 39566469 Free PMC article.
-
Exploiting natural killer group 2D receptors for CAR T-cell therapy.Future Oncol. 2017 Aug;13(18):1593-1605. doi: 10.2217/fon-2017-0102. Epub 2017 Jun 14. Future Oncol. 2017. PMID: 28613086 Review.
-
Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy.Acta Pharmacol Sin. 2018 Feb;39(2):167-176. doi: 10.1038/aps.2017.125. Epub 2017 Sep 7. Acta Pharmacol Sin. 2018. PMID: 28880014 Free PMC article. Review.
Cited by
-
Overcoming Target Driven Fratricide for T Cell Therapy.Front Immunol. 2018 Dec 12;9:2940. doi: 10.3389/fimmu.2018.02940. eCollection 2018. Front Immunol. 2018. PMID: 30619300 Free PMC article.
-
CD8+ T Cells in SARS-CoV-2 Induced Disease and Cancer-Clinical Perspectives.Front Immunol. 2022 Apr 1;13:864298. doi: 10.3389/fimmu.2022.864298. eCollection 2022. Front Immunol. 2022. PMID: 35432340 Free PMC article. Review.
-
Myelodysplastic syndrome and immunotherapy novel to next in-line treatments.Hum Vaccin Immunother. 2021 Aug 3;17(8):2602-2616. doi: 10.1080/21645515.2021.1898307. Epub 2021 May 3. Hum Vaccin Immunother. 2021. PMID: 33941042 Free PMC article. Review.
-
Epi-immunotherapy for cancers: rationales of epi-drugs in combination with immunotherapy and advances in clinical trials.Cancer Commun (Lond). 2022 Jun;42(6):493-516. doi: 10.1002/cac2.12313. Epub 2022 Jun 1. Cancer Commun (Lond). 2022. PMID: 35642676 Free PMC article. Review.
-
Immunotherapy in colorectal cancer: current achievements and future perspective.Int J Biol Sci. 2021 Sep 3;17(14):3837-3849. doi: 10.7150/ijbs.64077. eCollection 2021. Int J Biol Sci. 2021. PMID: 34671202 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
