Mycobacterium tuberculosis GroEL2 Modulates Dendritic Cell Responses
- PMID: 29133346
- PMCID: PMC5778359
- DOI: 10.1128/IAI.00387-17
Mycobacterium tuberculosis GroEL2 Modulates Dendritic Cell Responses
Abstract
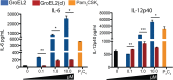
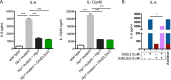
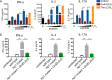
Mycobacterium tuberculosis successfully subverts the host immune response to promote disease progression. In addition to its known intracellular niche in macrophages, M. tuberculosis interferes with the functions of dendritic cells (DCs), which are the primary antigen-presenting cells of the immune system. We previously showed that M. tuberculosis dampens proinflammatory responses and impairs DC functions through the cell envelope-associated serine protease Hip1. Here we present data showing that M. tuberculosis GroEL2, a substrate of Hip1, modulates DC functions. The full-length GroEL2 protein elicited robust proinflammatory responses from DCs and promoted DC maturation and antigen presentation to T cells. In contrast, the cleaved form of GroEL2, which predominates in M. tuberculosis, was poorly immunostimulatory and was unable to promote DC maturation and antigen presentation. Moreover, DCs exposed to full-length, but not cleaved, GroEL2 induced strong antigen-specific gamma interferon (IFN-γ), interleukin-2 (IL-2), and IL-17A cytokine responses from CD4+ T cells. Moreover, the expression of cleaved GroEL2 in the hip1 mutant restored the robust T cell responses to wild-type levels, suggesting that proteolytic cleavage of GroEL2 allows M. tuberculosis to prevent optimal DC-T cell cross talk during M. tuberculosis infection.
Keywords: Mycobacterium tuberculosis; dendritic cells.
Copyright © 2018 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

