A Mucin-Like Protein of Planthopper Is Required for Feeding and Induces Immunity Response in Plants
- PMID: 29133370
- PMCID: PMC5761773
- DOI: 10.1104/pp.17.00755
A Mucin-Like Protein of Planthopper Is Required for Feeding and Induces Immunity Response in Plants
Abstract
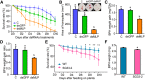
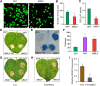
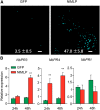
The brown planthopper, Nilaparvata lugens, is a pest that threatens rice (Oryza sativa) production worldwide. While feeding on rice plants, planthoppers secrete saliva, which plays crucial roles in nutrient ingestion and modulating plant defense responses, although the specific functions of salivary proteins remain largely unknown. We identified an N. lugens-secreted mucin-like protein (NlMLP) by transcriptome and proteome analyses and characterized its function, both in brown planthopper and in plants. NlMLP is highly expressed in salivary glands and is secreted into rice during feeding. Inhibition of NlMLP expression in planthoppers disturbs the formation of salivary sheaths, thereby reducing their performance. In plants, NlMLP induces cell death, the expression of defense-related genes, and callose deposition. These defense responses are related to Ca2+ mobilization and the MEK2 MAP kinase and jasmonic acid signaling pathways. The active region of NlMLP that elicits plant responses is located in its carboxyl terminus. Our work provides a detailed characterization of a salivary protein from a piercing-sucking insect other than aphids. Our finding that the protein functions in plant immune responses offers new insights into the mechanism underlying interactions between plants and herbivorous insects.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Atamian HS, Chaudhary R, Cin VD, Bao E, Girke T, Kaloshian I (2013) In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol Plant Microbe Interact 26: 67–74 - PubMed
-
- Axelsson MA, Asker N, Hansson GC (1998) O-Glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem 273: 18864–18870 - PubMed
-
- Backus EA, Serrano MS, Ranger CM (2005) Mechanisms of hopperburn: an overview of insect taxonomy, behavior, and physiology. Annu Rev Entomol 50: 125–151 - PubMed
-
- Bonaventure G. (2012) Perception of insect feeding by plants. Plant Biol (Stuttg) 14: 872–880 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

