Gradually Decreasing Starch Branching Enzyme Expression Is Responsible for the Formation of Heterogeneous Starch Granules
- PMID: 29133372
- PMCID: PMC5761781
- DOI: 10.1104/pp.17.01013
Gradually Decreasing Starch Branching Enzyme Expression Is Responsible for the Formation of Heterogeneous Starch Granules
Abstract
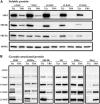
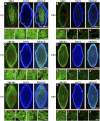
Rice (Oryza sativa) endosperm is mainly occupied by homogeneous polygonal starch from inside to outside. However, morphologically different (heterogeneous) starches have been identified in some rice mutants. How these heterogeneous starches form remains unknown. A high-amylose rice line (TRS) generated through the antisense inhibition of starch branching synthase I (SBEI) and SBEIIb contains four heterogeneous starches: polygonal, aggregate, elongated, and hollow starch; these starches are regionally distributed in the endosperm from inside to outside. Here, we investigated the relationship between SBE dosage and the morphological architecture of heterogeneous starches in TRS endosperm from the view of the molecular structure of starch. The results indicated that their molecular structures underwent regular changes, including gradually increasing true amylose content but decreasing amylopectin content and gradually increasing the ratio of amylopectin long chain but decreasing the ratio of amylopectin short chain. Granule-bound starch synthase I (GBSSI) amounts in the four heterogeneous starches were not significantly different from each other, but SBEI, SBEIIa, and SBEIIb showed a gradually decreasing trend. Further immunostaining analysis revealed that the gradually decreasing SBEs acting on the formation of the four heterogeneous granules were mainly due to the spatial distribution of the three SBEs in the endosperm. It was suggested that the decreased amylopectin in starch might remove steric hindrance and provide extra space for abundant amylose accumulation when the GBSSI amount was not elevated. Furthermore, extra amylose coupled with altered amylopectin structure possibly led to morphological changes in heterogeneous granules.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures





References
-
- Ao Z, Jane JL (2007) Characterization and modeling of the A- and B-granule starches of wheat, triticale, and barley. Carbohydr Polym 67: 46–55
-
- Blazek J, Gilbert EP (2011) Application of small-angle x-ray and neutron scattering techniques to the characterisation of starch structure: a review. Carbohydr Polym 85: 281–293
-
- Butardo VM, Fitzgerald MA, Bird AR, Gidley MJ, Flanagan BM, Larroque O, Resurreccion AP, Laidlaw HKC, Jobling SA, Morell MK, et al. (2011) Impact of down-regulation of starch branching enzyme IIb in rice by artificial microRNA- and hairpin RNA-mediated RNA silencing. J Exp Bot 62: 4927–4941 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

