Restoration of Rostral Ventrolateral Medulla Cystathionine- γ Lyase Activity Underlies Moxonidine-Evoked Neuroprotection and Sympathoinhibition in Diabetic Rats
- PMID: 29133386
- PMCID: PMC5771313
- DOI: 10.1124/jpet.117.243865
Restoration of Rostral Ventrolateral Medulla Cystathionine- γ Lyase Activity Underlies Moxonidine-Evoked Neuroprotection and Sympathoinhibition in Diabetic Rats
Abstract
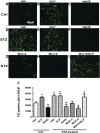
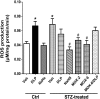
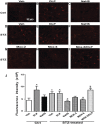
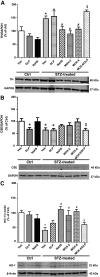
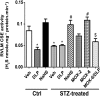
We recently demonstrated a fundamental role for cystathionine-γ lyase (CSE)-derived hydrogen sulfide (H2S) in the cardioprotective effect of the centrally acting drug moxonidine in diabetic rats. Whether a downregulated CSE/H2S system in the rostral ventrolateral medulla (RVLM) underlies neuronal oxidative stress and sympathoexcitation in diabetes has not been investigated. Along with addressing this question, we tested the hypothesis that moxonidine prevents the diabetes-evoked neurochemical effects by restoring CSE/H2S function within its major site of action, the RVLM. Ex vivo studies were performed on RVLM tissues of streptozotocin (55 mg/kg, i.p.) diabetic rats treated daily for 3 weeks with moxonidine (2 or 6 mg/kg; gavage), H2S donor sodium hydrosulfide (NaHS) (3.4 mg/kg, i.p.), CSE inhibitor DL-propargylglycine (DLP) (37.5 mg/kg, i.p.), a combination of DLP with moxonidine, or their vehicle. Moxonidine alleviated RVLM oxidative stress, neuronal injury, and increased tyrosine hydroxylase immunoreactivity (sympathoexcitation) by restoring CSE expression/activity as well as heme oxygenase-1 (HO-1) expression. A pivotal role for H2S in moxonidine-evoked neuroprotection is supported by the following: 1) NaHS replicated the moxonidine-evoked neuroprotection, and the restoration of RVLM HO-1 expression in diabetic rats; and 2) DLP abolished moxonidine-evoked neuroprotection in diabetic rats, and caused RVLM neurotoxicity, reminiscent of a diabetes-evoked neuronal phenotype, in healthy rats. These findings suggest a novel role for RVLM CSE/H2S/HO-1 in moxonidine-evoked neuroprotection and sympathoinhibition, and as a therapeutic target for developing new drugs for alleviating diabetes-evoked RVLM neurotoxicity and cardiovascular anomalies.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Abdel Moneim AE. (2015) The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis 30:935–942. - PubMed
-
- Bahniwal M, Little JP, Klegeris A. (2017) High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr Alzheimer Res 14:731–741. - PubMed
-
- Bakuridze K, Savli E, Gongadze N, Baş DB, Gepdiremen A. (2009) Protection in glutamate-induced neurotoxicity by imidazoline receptor agonist moxonidine. Int J Neurosci 119:1705–1717. - PubMed
-
- Biessels GJ, van der Heide LP, Kamal A, Bleys RL, Gispen WH. (2002) Ageing and diabetes: implications for brain function. Eur J Pharmacol 441:1–14. - PubMed
-
- Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, et al. (2004) Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology 25:101–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

