Repression of miR-31 by BCL6 stabilizes the helper function of human follicular helper T cells
- PMID: 29133396
- PMCID: PMC5715737
- DOI: 10.1073/pnas.1705364114
Repression of miR-31 by BCL6 stabilizes the helper function of human follicular helper T cells
Abstract
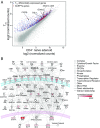
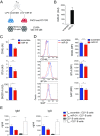
Follicular helper T cells (TFHs) are a key component of adaptive immune responses as they help antibody production by B cells. Differentiation and function of TFH cells are controlled by the master gene BCL6, but it is largely unclear how this transcription repressor specifies the TFH program. Here we asked whether BCL6 controlled helper function through down-regulation of specific microRNAs (miRNAs). We first assessed miRNA expression in TFH cells and defined a TFH-specific miRNA signature. We report that hsa-miR-31-5p (miR-31) is down-regulated in TFH; we showed that BCL6 suppresses miR-31 expression by binding to its promoter; and we demonstrated that miR-31 inhibits the expression of molecules that control T-helper function, such as CD40L and SAP. These findings identify a BCL6-initiated inhibitory circuit that stabilizes the follicular helper T cell program at least in part through the control of miRNA transcription. Although BCL6 controls TFH activity in human and mouse, the role of miR-31 is restricted to human TFH cell differentiation, reflecting a species specificity of the miR-31 action. Our findings highlight miR-31 as a possible target to modulate human T cell dependent antibody responses in the settings of infection, vaccination, or immune dysregulation.
Keywords: BCL6; RNA-seq; human CD4+ TFH; microRNA; transcriptional regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
CD25(+) Bcl6(low) T follicular helper cells provide help to maturing B cells in germinal centers of human tonsil.Eur J Immunol. 2015 Jan;45(1):298-308. doi: 10.1002/eji.201444911. Epub 2014 Oct 27. Eur J Immunol. 2015. PMID: 25263533 Free PMC article.
-
Genome-wide Analysis Identifies Bcl6-Controlled Regulatory Networks during T Follicular Helper Cell Differentiation.Cell Rep. 2016 Feb 23;14(7):1735-1747. doi: 10.1016/j.celrep.2016.01.038. Epub 2016 Feb 11. Cell Rep. 2016. PMID: 26876184 Free PMC article.
-
Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation.J Immunol. 2012 Apr 15;188(8):3734-44. doi: 10.4049/jimmunol.1103246. Epub 2012 Mar 16. J Immunol. 2012. PMID: 22427637 Free PMC article.
-
Follicular Helper T Cells.Annu Rev Immunol. 2016 May 20;34:335-68. doi: 10.1146/annurev-immunol-041015-055605. Epub 2016 Feb 22. Annu Rev Immunol. 2016. PMID: 26907215 Review.
-
Transcriptional regulation of follicular T-helper (Tfh) cells.Immunol Rev. 2013 Mar;252(1):139-45. doi: 10.1111/imr.12040. Immunol Rev. 2013. PMID: 23405901 Free PMC article. Review.
Cited by
-
Breast cancer tumor microenvironment affects Treg/IL-17-producing Treg/Th17 cell axis: Molecular and therapeutic perspectives.Mol Ther Oncolytics. 2023 Jan 11;28:132-157. doi: 10.1016/j.omto.2023.01.001. eCollection 2023 Mar 16. Mol Ther Oncolytics. 2023. PMID: 36816749 Free PMC article. Review.
-
Identification of Key Genes and Pathways in Osteoarthritis via Bioinformatic Tools: An Updated Analysis.Cartilage. 2021 Dec;13(1_suppl):1457S-1464S. doi: 10.1177/19476035211008975. Epub 2021 Apr 15. Cartilage. 2021. PMID: 33855867 Free PMC article.
-
Why is early-onset atrial fibrillation uncommon in patients with Duchenne muscular dystrophy? Insights from the mdx mouse.Cardiovasc Res. 2024 Apr 30;120(5):519-530. doi: 10.1093/cvr/cvae022. Cardiovasc Res. 2024. PMID: 38270932 Free PMC article.
-
Genome Editing With TALEN, CRISPR-Cas9 and CRISPR-Cas12a in Combination With AAV6 Homology Donor Restores T Cell Function for XLP.Front Genome Ed. 2022 May 23;4:828489. doi: 10.3389/fgeed.2022.828489. eCollection 2022. Front Genome Ed. 2022. PMID: 35677600 Free PMC article.
-
miRNA-Mediated Control of B Cell Responses in Immunity and SLE.Front Immunol. 2021 May 17;12:683710. doi: 10.3389/fimmu.2021.683710. eCollection 2021. Front Immunol. 2021. PMID: 34079558 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

