Locus Coeruleus Ablation Exacerbates Cognitive Deficits, Neuropathology, and Lethality in P301S Tau Transgenic Mice
- PMID: 29133432
- PMCID: PMC5761438
- DOI: 10.1523/JNEUROSCI.1483-17.2017
Locus Coeruleus Ablation Exacerbates Cognitive Deficits, Neuropathology, and Lethality in P301S Tau Transgenic Mice
Abstract
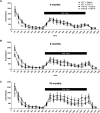
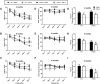
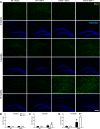
The brainstem locus coeruleus (LC) supplies norepinephrine to the forebrain and degenerates in Alzheimer's disease (AD). Loss of LC neurons is correlated with increased severity of other AD hallmarks, including β-amyloid (Aβ) plaques, tau neurofibrillary tangles, and cognitive deficits, suggesting that it contributes to the disease progression. Lesions of the LC in amyloid-based transgenic mouse models of AD exacerbate Aβ pathology, neuroinflammation, and cognitive deficits, but it is unknown how the loss of LC neurons affects tau-mediated pathology or behavioral abnormalities. Here we investigate the impact of LC degeneration in a mouse model of tauopathy by lesioning the LC of male and female P301S tau transgenic mice with the neurotoxin N-(2-chloroethyl)-N-ethyl-bromobenzylamine (DSP-4) starting at 2 months of age. By 6 months, deficits in hippocampal-dependent spatial (Morris water maze) and associative (contextual fear conditioning) memory were observed in lesioned P301S mice while performance remained intact in all other genotype and treatment groups, indicating that tau and LC degeneration act synergistically to impair cognition. By 10 months, the hippocampal neuroinflammation and neurodegeneration typically observed in unlesioned P301S mice were exacerbated by DSP-4, and mortality was also accelerated. These DSP-4-induced changes were accompanied by only a mild aggravation of tau pathology, suggesting that increased tau burden cannot fully account for the effects of LC degeneration. Combined, these experiments demonstrate that loss of LC noradrenergic neurons exacerbates multiple phenotypes caused by pathogenic tau, and provides complementary data to highlight the dual role LC degeneration has on both tau and Aβ pathologies in AD.SIGNIFICANCE STATEMENT Elucidating the mechanisms underlying AD is crucial to developing effective diagnostics and therapeutics. The degeneration of the LC and loss of noradrenergic transmission have been recognized as ubiquitous events in AD pathology, and previous studies demonstrated that LC lesions exacerbate pathology and cognitive deficits in amyloid-based mouse models. Here, we reveal a complementary role of LC degeneration on tau-mediated aspects of the disease by using selective lesions of the LC and the noradrenergic system to demonstrate an exacerbation of cognitive deficits, neuroinflammation, neurodegeneration in a transgenic mouse model of tauopathy. Our data support an integral role for the LC in modulating the severity of both canonical AD-associated pathologies, as well as the detrimental consequences of LC degeneration during disease progression.
Keywords: DSP-4; hippocampus; locus coeruleus; neuroinflammation; norepinephrine; tau.
Copyright © 2018 the authors 0270-6474/18/380074-19$15.00/0.
Figures









Comment in
-
Commentary: Locus Coeruleus Ablation Exacerbates Cognitive Deficits, Neuropathology, and Lethality in P301S Tau Transgenic Mice.Front Neurosci. 2018 Jun 6;12:401. doi: 10.3389/fnins.2018.00401. eCollection 2018. Front Neurosci. 2018. PMID: 29928191 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
