Elevated HuR in Pancreas Promotes a Pancreatitis-Like Inflammatory Microenvironment That Facilitates Tumor Development
- PMID: 29133460
- PMCID: PMC5770537
- DOI: 10.1128/MCB.00427-17
Elevated HuR in Pancreas Promotes a Pancreatitis-Like Inflammatory Microenvironment That Facilitates Tumor Development
Abstract
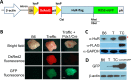
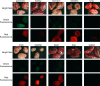
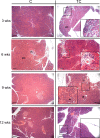
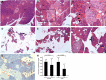
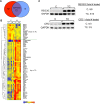
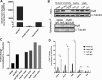
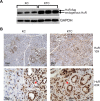
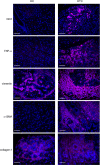
Human antigen R (ELAVL1; HuR) is perhaps the best-characterized RNA-binding protein. Through its overexpression in various tumor types, HuR promotes posttranscriptional regulation of target genes in multiple core signaling pathways associated with tumor progression. The role of HuR overexpression in pancreatic tumorigenesis is unknown and led us to explore the consequences of HuR overexpression using a novel transgenic mouse model that has a >2-fold elevation of pancreatic HuR expression. Histologically, HuR-overexpressing pancreas displays a fibroinflammatory response and other pathological features characteristic of chronic pancreatitis. This pathology is reflected in changes in the pancreatic gene expression profile due, in part, to genes whose expression changes as a consequence of direct binding of their respective mRNAs to HuR. Older mice develop pancreatic steatosis and severe glucose intolerance. Elevated HuR cooperated with mutant K-rasG12D to result in a 3.4-fold increase in pancreatic ductal adenocarcinoma (PDAC) incidence compared to PDAC presence in K-rasG12D alone. These findings implicate HuR as a facilitator of pancreatic tumorigenesis, especially in the setting of inflammation, and a novel therapeutic target for pancreatitis treatment.
Keywords: HuR; cancer; inflammation; pancreas.
Copyright © 2018 American Society for Microbiology.
Figures








References
-
- Blanco FF, Jimbo M, Wulfkuhle J, Gallagher I, Deng J, Enyenihi L, Meisner-Kober N, Londin ER, Rigoutsos I, Sawicki JA, Risud MV, Witkiewicz AK, McCue PA, Jiang W, Rui H, Yeo CJ, Petricoin EF, Winter JM, Brody JR. 2016. The mRNA-binding protein HuR promotes hypoxia-induced chemoresistance through posttranscriptional regulation of the proto-oncogene PIM1 in pancreatic cancer cells. Oncogene 35:2529–2541. doi: 10.1038/onc.2015.325. - DOI - PMC - PubMed
-
- Costantino CL, Witkiewicz AK, Kuwano Y, Cozzitorto JA, Kennedy EP, Dasgupta A, Keen JC, Yeo CJ, Gorospe M, Brody JR. 2009. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res 69:4567–4572. doi: 10.1158/0008-5472.CAN-09-0371. - DOI - PMC - PubMed
-
- Lal S, Burkhart RA, Bhattacharjee V, Beeharry N, Londin ER, Cozzitorto JA, Romeo C, Jimbo M, Norris ZA, Yeo CJ, Sawicki JA, Winter JM, Rigoutsos I, Yen TJ, Brody JR. 2014. HuR post-transcriptionally regulates WEE1: implications for the DNA damage response in pancreatic cancer cells. Cancer Res 74:1128–1140. doi: 10.1158/0008-5472.CAN-13-1915. - DOI - PMC - PubMed
-
- McAllister F, Pineda DM, Jimbo M, Lal S, Burkhart RA, Moughan J, Winter KA, Abdelmohsen K, Gorospe M, Acosta AdJ, Lankapalli RH, Winter JM, Yeo CJ, Witkiewicz AK, Iacobuzio-Donahue CA, Laheru D, Brody JR. 2014. dCK expression correlates with 5-fluorouracil efficacy and HuR cytoplasmic expression in pancreatic cancer. Cancer Biol Ther 15:688–698. doi: 10.4161/cbt.28413. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
