Pharmacokinetics and Exposure-Response Analysis of RG7667, a Combination of Two Anticytomegalovirus Monoclonal Antibodies, in a Phase 2a Randomized Trial To Prevent Cytomegalovirus Infection in High-Risk Kidney Transplant Recipients
- PMID: 29133549
- PMCID: PMC5786805
- DOI: 10.1128/AAC.01108-17
Pharmacokinetics and Exposure-Response Analysis of RG7667, a Combination of Two Anticytomegalovirus Monoclonal Antibodies, in a Phase 2a Randomized Trial To Prevent Cytomegalovirus Infection in High-Risk Kidney Transplant Recipients
Abstract
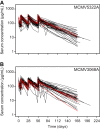
RG7667, a novel combination of two anticytomegalovirus (anti-CMV) monoclonal IgG1 antibodies (MCMV5322A and MCMV3068A), was designed to block CMV entry into host cells. It was developed as a potential therapy for preventing CMV infection and disease in transplant recipients. RG7667 was assessed for preventing CMV infection in a phase 2a trial with CMV-seronegative recipients of kidney transplants from CMV-seropositive donors. The patients received 4 intravenous doses of RG7667 (10 mg/kg of body weight of each antibody, n = 60) or placebo (n = 60) at the time of the transplant and at 1, 4, and 8 weeks after the transplant. Serum samples were collected for pharmacokinetic (PK) analysis and antidrug antibody (ADA) evaluation. To guide future dose selection, the relationships between RG7667 exposure and pharmacological activity were evaluated. MCMV5322A and MCMV3068A exposures were confirmed in all RG7667-treated patients. Mean clearances for MCMV5322A and MCMV3068A were 2.97 and 2.65 ml/day/kg, respectively, and the terminal half-lives of MCMV5322A and MCMV3068A were 26.9 and 27.4 days, respectively. The ADA incidence was low and was not associated with lower drug exposure. Patients with RG7667 or component antibody exposures greater than the respective median values had a lower incidence of viremia at 12 weeks and 24 weeks after transplantation and a longer delayed time to detectable CMV viremia than patients with exposures less than the median values. MCMV5322A and MCMV3068A exhibited expected IgG1 PK profiles in high-risk kidney transplant recipients, consistent with the earlier PK behavior of RG7667 in healthy subjects. Higher drug exposure was associated with better anti-CMV pharmacological activity. (This study has been registered at ClinicalTrials.gov under identifier NCT01753167.).
Keywords: RG7667; cytomegalovirus; kidney transplantation; monoclonal antibodies; pharmacokinetics.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Snydman DR, Werner BG, Heinze-Lacey B, Berardi VP, Tilney NL, Kirkman RL, Milford EL, Cho SI, Bush HL, Levey AS, Strom TB, Carpenter CB, Levey RH, Harmon WE, Zimmerman CE II, Shapiro ME, Steinman T, LoGerfo F, Idelson B, Schröter GPJ, Levin MJ, McIver J, Leszczynski J, Grady GF. 1987. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med 317:1049–1054. doi: 10.1056/NEJM198710223171703. - DOI - PubMed
-
- Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD, Valganciclovir Solid Organ Transplant Study Group. 2004. Efficacy and safety of valganciclovir versus oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 4:611–620. doi: 10.1111/j.1600-6143.2004.00382.x. - DOI - PubMed
-
- Razonable RR, Rivero A, Rodriguez A, Wilson J, Daniels J, Jenkins G, Larson T, Hellinger WC, Spivey JR, Paya CV. 2001. Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxis with oral ganciclovir. J Infect Dis 184:1461–1464. doi: 10.1086/324516. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

