Pharmacokinetics and Drug-Drug Interactions of Lopinavir-Ritonavir Administered with First- and Second-Line Antituberculosis Drugs in HIV-Infected Children Treated for Multidrug-Resistant Tuberculosis
- PMID: 29133558
- PMCID: PMC5786799
- DOI: 10.1128/AAC.00420-17
Pharmacokinetics and Drug-Drug Interactions of Lopinavir-Ritonavir Administered with First- and Second-Line Antituberculosis Drugs in HIV-Infected Children Treated for Multidrug-Resistant Tuberculosis
Abstract
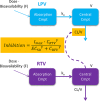
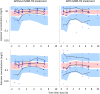
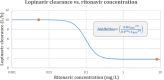
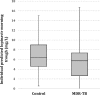
Lopinavir-ritonavir forms the backbone of current first-line antiretroviral regimens in young HIV-infected children. As multidrug-resistant (MDR) tuberculosis (TB) frequently occurs in young children in high-burden TB settings, it is important to identify potential interactions between MDR-TB treatment and lopinavir-ritonavir. We describe the pharmacokinetics of and potential drug-drug interactions between lopinavir-ritonavir and drugs routinely used for MDR-TB treatment in HIV-infected children. A combined population pharmacokinetic model was developed to jointly describe the pharmacokinetics of lopinavir and ritonavir in 32 HIV-infected children (16 with MDR-TB receiving treatment with combinations of high-dose isoniazid, pyrazinamide, ethambutol, ethionamide, terizidone, a fluoroquinolone, and amikacin and 16 without TB) who were established on a lopinavir-ritonavir-containing antiretroviral regimen. One-compartment models with first-order absorption and elimination for both lopinavir and ritonavir were combined into an integrated model. The dynamic inhibitory effect of the ritonavir concentration on lopinavir clearance was described using a maximum inhibition model. Even after adjustment for the effect of body weight with allometric scaling, a large variability in lopinavir and ritonavir exposure, together with strong correlations between the pharmacokinetic parameters of lopinavir and ritonavir, was detected. MDR-TB treatment did not have a significant effect on the bioavailability, clearance, or absorption rate constants of lopinavir or ritonavir. Most children (81% of children with MDR-TB, 88% of controls) achieved therapeutic lopinavir trough concentrations (>1 mg/liter). The coadministration of lopinavir-ritonavir with drugs routinely used for the treatment of MDR-TB was found to have no significant effect on the key pharmacokinetic parameters of lopinavir or ritonavir. These findings should be considered in the context of the large interpatient variability found in the present study and the study's modest sample size.
Keywords: Mycobacterium tuberculosis; antiretroviral agents; drug interactions; human immunodeficiency virus; multidrug resistance; pediatric drug therapy; pediatric infectious disease; pharmacokinetics; population pharmacokinetics.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- World Health Organization. 2013. Treatment of children living with HIV. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/topics/paediatric/en/.
-
- World Health Organization. 2015. Tuberculosis. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs104/en/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

