Quantitative analysis of multilayer organization of proteins and RNA in nuclear speckles at super resolution
- PMID: 29133588
- PMCID: PMC5769577
- DOI: 10.1242/jcs.206854
Quantitative analysis of multilayer organization of proteins and RNA in nuclear speckles at super resolution
Abstract
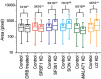
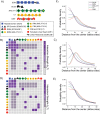
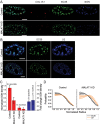
Nuclear speckles are self-assembled organelles composed of RNAs and proteins. They are proposed to act as structural domains that control distinct steps in gene expression, including transcription, splicing and mRNA export. Earlier studies identified differential localization of a few components within the speckles. It was speculated that the spatial organization of speckle components might contribute directly to the order of operations that coordinate distinct processes. Here, by performing multi-color structured illumination microscopy, we characterized the multilayer organization of speckles at a higher resolution. We found that SON and SC35 (also known as SRSF2) localize to the central region of the speckle, whereas MALAT1 and small nuclear (sn)RNAs are enriched at the speckle periphery. Coarse-grained simulations indicate that the non-random organization arises due to the interplay between favorable sequence-encoded intermolecular interactions of speckle-resident proteins and RNAs. Finally, we observe positive correlation between the total amount of RNA present within a speckle and the speckle size. These results imply that speckle size may be regulated to accommodate RNA accumulation and processing. Accumulation of RNA from various actively transcribed speckle-associated genes could contribute to the observed speckle size variations within a single cell.
Keywords: Long noncoding RNA; Nuclear domain; Splicing factor; Sub-nuclear compartmentalization.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsS.M.F. is an employee of Ionis Pharmaceuticals and receives salary from the company.
Figures



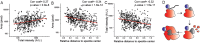
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

