Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study)
- PMID: 29133604
- PMCID: PMC5777572
- DOI: 10.1161/CIRCULATIONAHA.117.032038
Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study)
Abstract
Background: Canagliflozin is a sodium glucose cotransporter 2 inhibitor that significantly reduces the composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke in patients with type 2 diabetes mellitus and elevated cardiovascular risk. The comparative effects among participants with and without a history of cardiovascular disease (secondary versus primary prevention) were prespecified for evaluation.
Methods: The CANVAS Program (Canagliflozin Cardiovascular Assessment Study) randomly assigned 10 142 participants with type 2 diabetes mellitus to canagliflozin or placebo. The primary prevention cohort comprised individuals ≥50 years of age with ≥2 risk factors for cardiovascular events but with no prior cardiovascular event, and the secondary prevention cohort comprised individuals ≥30 years of age with a prior cardiovascular event. The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Secondary outcomes included heart failure hospitalization and a renal composite (40% reduction in estimated glomerular filtration rate, renal replacement therapy, or renal death).
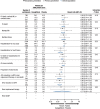
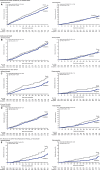
Results: Primary prevention participants (N=3486; 34%) were younger (63 versus 64 years of age), were more often female (45% versus 31%), and had a longer duration of diabetes mellitus (14 versus 13 years) compared with secondary prevention participants (N=6656; 66%). The primary end point event rate was higher in the secondary prevention group compared with the primary prevention group (36.9 versus 15.7/1000 patient-years, P<0.001). In the total cohort, the primary end point was reduced with canagliflozin compared with placebo (26.9 versus 31.5/1000 patient-years; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.75-0.97; P<0.001 for noninferiority, P=0.02 for superiority) with no statistical evidence of heterogeneity (interaction P value=0.18) between the primary (HR, 0.98; 95% CI, 0.74-1.30) and secondary prevention (HR, 0.82; 95% CI, 0.72-0.95) cohorts. Renal outcomes (HR, 0.59; 95% CI, 0.44-0.79 versus HR, 0.63; 95% CI, 0.39-1.02; interaction P value=0.73) and heart failure hospitalization (HR, 0.68; 95% CI, 0.51-0.90 versus HR, 0.64; 95% CI, 0.35-1.15; interaction P value=0.91) were similarly reduced in the secondary and primary prevention cohorts, respectively. Lower extremity amputations were similarly increased in the secondary and primary prevention cohorts (HR, 2.07; 95% CI, 1.43-3.00 versus HR, 1.52; 95% CI, 0.70-3.29; interaction P value=0.63).
Conclusions: Patients with type 2 diabetes mellitus and prior cardiovascular events had higher rates of cardiovascular outcomes compared with the primary prevention patients. Canagliflozin reduced cardiovascular and renal outcomes with no statistical evidence of heterogeneity of the treatment effect across the primary and secondary prevention groups. Additional studies will provide further insights into the effects of canagliflozin in these patient populations.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifiers: NCT01032629 and NCT01989754.
Keywords: canagliflozin; clinical trial; diabetes mellitus; primary prevention; secondary prevention.
© 2017 The Authors.
Figures


Comment in
-
Canagliflozin: Cui Bono?Circulation. 2018 Jan 23;137(4):335-337. doi: 10.1161/CIRCULATIONAHA.117.032198. Epub 2017 Nov 13. Circulation. 2018. PMID: 29133603 No abstract available.
-
Diabetes: Further insights into SGLT2 inhibitors.Nat Rev Cardiol. 2018 Jan;15(1):2-3. doi: 10.1038/nrcardio.2017.198. Epub 2017 Nov 30. Nat Rev Cardiol. 2018. PMID: 29188808 No abstract available.
References
-
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. - PubMed
-
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. - PubMed
-
- Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, Desai M, Shaw W, Jiang J, Vercruysse F, Meininger G, Matthews D. Rationale, design, and baseline characteristics of the CANagliflozin cardioVascular Assessment Study (CANVAS): a randomized placebo-controlled trial. Am Heart J. 2013;166:217.e11–223.e11. doi: 10.1016/j.ahj.2013.05.007. - PubMed
-
- Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, Meininger G, Erondu N, Desai M, Shaw W, Vercruysse F, Yee J, Deng H, de Zeeuw D CANVAS-R Trial Collaborative Group. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19:387–393. doi: 10.1111/dom.12829. - PMC - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, Fulcher G, Erondu N, Desai M, Shaw W, Law G, Walton MK, Rosenthal N, de Zeeuw D, Matthews DR CANVAS Program Collaborative Group. Optimizing the analysis strategy for the CANVAS Program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab. 2017;19:926–935. doi: 10.1111/dom.12924. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

