Motivational interviewing and urine cotinine feedback to stop passive smoke exposure in children predisposed to asthma: a randomised controlled trial
- PMID: 29133798
- PMCID: PMC5684321
- DOI: 10.1038/s41598-017-15158-2
Motivational interviewing and urine cotinine feedback to stop passive smoke exposure in children predisposed to asthma: a randomised controlled trial
Abstract
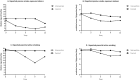
We tested the effectiveness of a program consisting of motivational interviewing (MI) and feedback of urine cotinine to stop passive smoking (PS) in children at risk for asthma. Fifty-eight families with children 0-13 years with a high risk of asthma and PS exposure were randomised in a one-year follow-up study. The intervention group received the intervention program during 6 sessions (1/month) and the control group received measurements (questionnaires, urine cotinine, and lung function) only. The primary outcome measure was the percentage of families stopping PS (parental report verified and unverified with the child's urine cotinine concentration <10 μg/l) in children during the intervention program. The analyses were performed with Mixed Logistic Regression. After 6 months, a significant group difference was observed for the unverified parental report of stopping PS in children: 27% of parents in the intervention group versus 7% in the control group. For the verified parental report, the difference was similar (23% versus 7%) but was not statistically significant. Despite a limited sample size, the results suggest that the intervention program is probably an effective strategy to stop PS in children. A program longer than 6 months might be necessary for a longer lasting intervention effect.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

