Eph-B4 regulates adaptive venous remodeling to improve arteriovenous fistula patency
- PMID: 29133876
- PMCID: PMC5684317
- DOI: 10.1038/s41598-017-13071-2
Eph-B4 regulates adaptive venous remodeling to improve arteriovenous fistula patency
Abstract
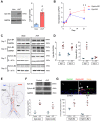
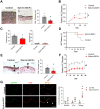
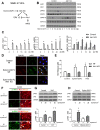
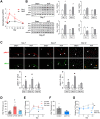
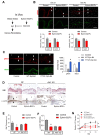
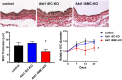
Low rates of arteriovenous fistula (AVF) maturation prevent optimal fistula use for hemodialysis; however, the mechanism of venous remodeling in the fistula environment is not well understood. We hypothesized that the embryonic venous determinant Eph-B4 mediates AVF maturation. In human AVF and a mouse aortocaval fistula model, Eph-B4 protein expression increased in the fistula vein; expression of the arterial determinant Ephrin-B2 also increased. Stimulation of Eph-B-mediated signaling with Ephrin-B2/Fc showed improved fistula patency with less wall thickness. Mutagenesis studies showed that tyrosine-774 is critical for Eph-B4 signaling and administration of inactive Eph-B4-Y774F increased fistula wall thickness. Akt1 expression also increased in AVF; Akt1 knockout mice showed reduced fistula diameter and wall thickness. In Akt1 knockout mice, stimulation of Eph-B signaling with Ephrin-B2/Fc showed no effect on remodeling. These results show that AVF maturation is associated with acquisition of dual arteriovenous identity; increased Eph-B activity improves AVF patency. Inhibition of Akt1 function abolishes Eph-B-mediated venous remodeling suggesting that Eph-B4 regulates AVF venous adaptation through an Akt1-mediated mechanism.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Gibson KD, et al. Vascular access survival and incidence of revisions: a comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg. 2001;34:694–700. doi: 10.1067/mva.2001.117890. - DOI - PubMed
-
- Besarab A, Ravani P, Spergel LM, Roy-Chaudhury P, Asif A. The native arteriovenous fistula in 2007. Research needs. J Nephrol. 2007;20:668–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

