Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration
- PMID: 29133913
- PMCID: PMC5684213
- DOI: 10.1038/s41598-017-14970-0
Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration
Erratum in
-
Author Correction: Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration.Sci Rep. 2018 Mar 6;8(1):4203. doi: 10.1038/s41598-018-21306-z. Sci Rep. 2018. PMID: 29511220 Free PMC article.
Abstract
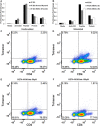
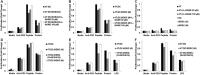
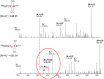
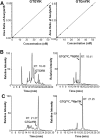
Myeloid derived suppressor cells (MDSC) produce nitric oxide (NO) and inhibit dendritic cell (DC) immune responses in cancer. DCs present cancer cell antigens to CD4+ T cells through Jak-STAT signal transduction. In this study, NO donors (SNAP and DETA-NONOate) inhibited DC antigen presentation. As expected, MDSC isolated from peripheral blood mononuclear cells (PBMC) from cancer patients produced high NO levels. We hypothesized that NO producing MDSC in tumor-bearing hosts would inhibit DC antigen presentation. Antigen presentation from DCs to CD4+ T cells (T cell receptor transgenic OT-II) was measured via a [3H]-thymidine incorporation proliferation assay. MDSC from melanoma tumor models decreased the levels of proliferation more than pancreatic cancer derived MDSC. T cell proliferation was restored when MDSC were treated with inhibitors of inducible nitric oxide synthase (L-NAME and NCX-4016). A NO donor inhibited OT II T cell receptor recognition of OT II specific tetramers, thus serving as a direct measure of NO inhibition of antigen presentation. Our group has previously demonstrated that STAT1 nitration also mediates MDSC inhibitory effects on immune cells. Therefore, a novel liquid chromatography-tandem mass spectrometry assay demonstrated that nitration of the STAT1-Tyr701 occurs in PBMC derived from both pancreatic cancer and melanoma patients.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

