Fe(OTf)3-catalysed Friedel-Crafts reaction of benzenoid arenes with α,β-unsaturated carbonyl compounds: easy access to 1,1-diarylalkanes
- PMID: 29134078
- PMCID: PMC5666261
- DOI: 10.1098/rsos.170748
Fe(OTf)3-catalysed Friedel-Crafts reaction of benzenoid arenes with α,β-unsaturated carbonyl compounds: easy access to 1,1-diarylalkanes
Abstract
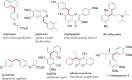
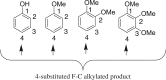
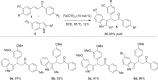
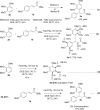
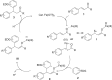
A simple and efficient method for the synthesis of 1,1-diarylalkanes via the Friedel-Crafts-type alkylation reaction of electron-rich arenes with cinnamic acid ester derivatives or chalcones is reported. Iron triflate has been found to be the best catalyst for the Friedel-Crafts-type alkylation reaction with α,β-unsaturated carbonyl compounds. This reaction afforded β,β-diaryl carbonyl compounds in good yields (65-93%) and with excellent regioselectivities. Remarkably, this method is also compatible with a variety of indoles to provide 3-indolyl-aryl carbonyl compounds in excellent yields. Great efforts have been made to deduce a plausible reaction mechanism based on isotopic labelling experiments.
Keywords: 1,1-diarylalkane; Friedel–Crafts alkylation; iron triflate; β,β-diaryl carbonyl compounds.
Conflict of interest statement
The authors declare no competing interests.
Figures
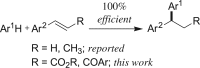
References
-
- Rueping M, Nachtsheim BJ. 2010. A review of new developments in the Friedel–Crafts alkylation—from green chemistry to asymmetric catalysis. Beilstein J. Org. Chem. 6, 1–31. (doi:10.3762/bjoc.6.6) - DOI - PMC - PubMed
-
- Kumar R, Van der Eycken EV. 2013. Recent approaches for C–C bond formation via direct dehydrative coupling strategies. Chem. Soc. Rev. 42, 1121–1146. (doi:10.1039/C2CS35397K) - DOI - PubMed
-
- Bandini M, Melloni A, Umani-Ronchi A. 2004. New catalytic approaches in the stereoselective Friedel–Crafts alkylation reaction . Angew. Chem. Int. Ed. 43, 550–556. (doi:10.1002/anie.200301679) - DOI - PubMed
-
- Naredla RR, Klumpp DA. 2013. Contemporary carbocation chemistry: applications in organic synthesis. Chem. Rev. 113, 6905–6948. (doi:10.1021/cr4001385) - DOI - PubMed
-
- You S-L, Cai Q, Zeng M. 2009. Chiral Brønsted acid catalyzed Friedel–Crafts alkylation reactions. Chem. Soc. Rev. 38, 2190–2201. (doi:10.1039/b817310a) - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
