Gene delivery ability of polyethylenimine and polyethylene glycol dual-functionalized nanographene oxide in 11 different cell lines
- PMID: 29134085
- PMCID: PMC5666268
- DOI: 10.1098/rsos.170822
Gene delivery ability of polyethylenimine and polyethylene glycol dual-functionalized nanographene oxide in 11 different cell lines
Abstract
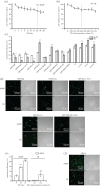
We recently developed a polyethylenimine (PEI) and polyethylene glycol (PEG) dual-functionalized reduced graphene oxide (GO) (PEG-nrGO-PEI, RGPP) for high-efficient gene delivery in HepG2 and Hela cell lines. To evaluate the feasibility and applicability of RGPP as a gene delivery carrier, we here assessed the transfection efficiency of RGPP on gene plasmids and siRNA in 11 different cell lines. Commercial polyalkyleneimine cation transfection reagent (TR) was used as comparison. In HepG2 cells, RGPP exhibited much stronger delivery ability for siRNA and large size plasmids than TR. For green fluorescent protein (GFP) plasmid, RGPP showed about 47.1% of transfection efficiency in primary rabbit articular chondrocytes, and about 27% of transfection efficiency in both SH-SY5Y and A549 cell lines. RGPP exhibited about 37.2% of GFP plasmid transfection efficiency in EMT6 cells and about 26.0% of GFP plasmid transfection efficiency in LO2 cells, but induced about 33% of cytotoxicity in both cell lines. In 4T1 and H9C2 cell lines, RGPP had less than 10% of GFP plasmid transfection efficiency. Collectively, RGPP is a potential nano-carrier for high-efficiency gene delivery, and needs to be further optimized for different cell lines.
Keywords: gene delivery; graphene oxide; polyethylene glycol; polyethylenimine; transfection efficiency.
Conflict of interest statement
We declare we have no competing interests.
Figures




References
-
- Wong JK, Mohseni R, Hamidieh AA, Maclaren RE, Habib N, Seifalian AM. 2017. Will nanotechnology bring new hope for gene delivery? Trends Biotechnol. 35, 434–451. (doi:10.1016/j.tibtech.2016.12.009) - DOI - PubMed
-
- Mulligan RC. 1993. The basic science of gene therapy. Science 260, 926–932. (doi:10.1126/science.8493530) - DOI - PubMed
-
- Dyer MR, Herrling PL. 2000. Progress and potential for gene-based medicines. Mol. Ther. 1, 213–224. (doi:10.1006/mthe.2000.0044) - DOI - PubMed
-
- Onuki R, Nagasaki A, Kawasaki H, Baba T, Uyeda TQ, Taira K. 2002. Confirmation by FRET in individual living cells of the absence of significant amyloid β-mediated caspase 8 activation. Proc. Natl Acad. Sci. USA 99, 14 716–14 721. (doi:10.1073/pnas.232177599) - DOI - PMC - PubMed
-
- Takemoto K, Nagai T, Miyawaki A, Miura M. 2003. Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J. Cell Biol. 160, 235–243. (doi:10.1083/jcb.200207111) - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
