Acute Administration of the Nonpathogenic, Saprophytic Bacterium, Mycobacterium vaccae, Induces Activation of Serotonergic Neurons in the Dorsal Raphe Nucleus and Antidepressant-Like Behavior in Association with Mild Hypothermia
- PMID: 29134419
- PMCID: PMC11481832
- DOI: 10.1007/s10571-017-0564-3
Acute Administration of the Nonpathogenic, Saprophytic Bacterium, Mycobacterium vaccae, Induces Activation of Serotonergic Neurons in the Dorsal Raphe Nucleus and Antidepressant-Like Behavior in Association with Mild Hypothermia
Abstract
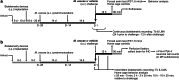
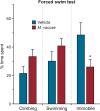
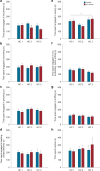
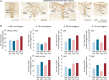
Peripheral immune activation can have profound physiologic and behavioral effects. One mechanism through which immune activation may affect physiology and behavior is through actions on brainstem neuromodulatory systems, such as serotonergic systems. To test this hypothesis, in Experiment 1, adult male BALB/c mice were implanted with telemetric recording devices and then immunized with Mycobacterium vaccae NCTC 11659 (0.1 mg, s.c.; Days - 28, - 14; N = 36). On Day 1, mice received an acute challenge with M. vaccae (0.1 mg, s.c.) or borate-buffered saline vehicle. Core body temperature and locomotor activity recordings were conducted during a 36 h period beginning 24 h prior to challenge; 12 h following acute challenge, mice were either tested in a 6-min forced swim test, or served as home cage controls (n = 9 per group). In Experiment 2, the protocol was repeated, but with the aim of assessing c-Fos expression in brainstem serotonergic neurons, assessed 90 min following exposure to forced swim (N = 32; n = 8 per group). In Experiment 1, acute M. vaccae challenge in M. vaccae-immunized mice, relative to vehicle-challenged controls, decreased locomotor activity and core body temperature measured 3 h following challenge, as measured by continuous telemetric recordings, and decreased immobility in the forced swim test measured 12 h following challenge. In Experiment 2, acute M. vaccae challenge in M. vaccae-immunized mice decreased home cage locomotion, in alignment with findings in Experiment 1, as measured by video-based behavioral analysis, and, among mice exposed to the forced swim test, increased c-Fos expression in subsets of serotonergic neurons within the dorsal raphe nucleus (DR) measured 13.5 h following challenge. Together, these data are consistent with the hypothesis that acute peripheral immune activation with a heat-killed preparation of M. vaccae transiently induces mild hypothermia in association with suppression of locomotor activity, activates subsets of serotonergic neurons in the DR, and induces antidepressant-like behavioral responses.
Keywords: Actinobacteria; Antidepressant; Body temperature; Hypothermia; M. vaccae; Mycobacterium vaccae.
Conflict of interest statement
Christopher A. Lowry serves on the Scientific Advisory Board of Immodulon Therapeutics Ltd. The remaining authors declare that they have no conflict of interest.
Figures



References
-
- Busch M, Chourbaji S, Dammann P, Haemisch A, Jirkof P, Oehlert P, Osterkamp A, Ott S, Peters S, Spekl K, Tsai PP (2014) Tiergerechte Haltung von Labormäusen: Ausschuss für Tiergerechte Laborierhaltung. GV-SOLAS, Gesellschaft für Versuchstierkunde
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

