Aminoglycoside Damage and Hair Cell Regeneration in the Chicken Utricle
- PMID: 29134476
- PMCID: PMC5783928
- DOI: 10.1007/s10162-017-0646-4
Aminoglycoside Damage and Hair Cell Regeneration in the Chicken Utricle
Erratum in
-
Correction to: Aminoglycoside Damage and Hair Cell Regeneration in the Chicken Utricle.J Assoc Res Otolaryngol. 2018 Feb;19(1):31. doi: 10.1007/s10162-017-0650-8. J Assoc Res Otolaryngol. 2018. PMID: 29299694 Free PMC article.
-
Correction to: Aminoglycoside Damage and Hair Cell Regeneration in the Chicken Utricle.J Assoc Res Otolaryngol. 2018 Aug;19(4):479-481. doi: 10.1007/s10162-018-0667-7. J Assoc Res Otolaryngol. 2018. PMID: 30076555 Free PMC article.
Abstract
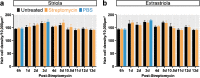
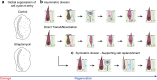
In this study, we present a systematic characterization of hair cell loss and regeneration in the chicken utricle in vivo. A single unilateral surgical delivery of streptomycin caused robust decline of hair cell numbers in striolar as well as extrastriolar regions, which in the striola was detected very early, 6 h post-insult. During the initial 12 h of damage response, we observed global repression of DNA replication, in contrast to the natural, mitotic hair cell production in undamaged control utricles. Regeneration of hair cells in striolar and extrastriolar regions occurred via high rates of asymmetric supporting cell divisions, accompanied by delayed replenishment by symmetric division. While asymmetric division of supporting cells is the main regenerative response to aminoglycoside damage, the detection of symmetric divisions supports the concept of direct transdifferentiation where supporting cells need to be replenished after their phenotypic conversion into new hair cells. Supporting cell divisions appear to be well coordinated because total supporting cell numbers throughout the regenerative process were invariant, despite the initial large-scale loss of hair cells. We conclude that a single ototoxic drug application provides an experimental framework to study the precise onset and timing of utricle hair cell regeneration in vivo. Our findings indicate that initial triggers and signaling events occur already within a few hours after aminoglycoside exposure. Direct transdifferentiation and asymmetric division of supporting cells to generate new hair cells subsequently happen largely in parallel and persist for several days.
Keywords: EdU; S-phase; cell cycle; inner ear; otic; vestibular.
Figures




Similar articles
-
Cytoskeletal protein mRNA expression in the chick utricle after treatment in vitro with aminoglycoside antibiotics: effects of insulin, iron chelators and cyclic nucleotides.Brain Res. 2000 Jul 21;871(2):319-32. doi: 10.1016/s0006-8993(00)02488-4. Brain Res. 2000. PMID: 10899298
-
YAP Mediates Hair Cell Regeneration in Balance Organs of Chickens, But LATS Kinases Suppress Its Activity in Mice.J Neurosci. 2020 May 13;40(20):3915-3932. doi: 10.1523/JNEUROSCI.0306-20.2020. Epub 2020 Apr 27. J Neurosci. 2020. PMID: 32341094 Free PMC article.
-
Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity.Hear Res. 2009 Jan;247(1):17-26. doi: 10.1016/j.heares.2008.08.010. Epub 2008 Sep 7. Hear Res. 2009. PMID: 18809482 Free PMC article.
-
Functional recovery in the avian ear after hair cell regeneration.Audiol Neurootol. 1999 Nov-Dec;4(6):286-302. doi: 10.1159/000013853. Audiol Neurootol. 1999. PMID: 10516389 Review.
-
Ongoing cell death and immune influences on regeneration in the vestibular sensory organs.Ann N Y Acad Sci. 2001 Oct;942:34-45. doi: 10.1111/j.1749-6632.2001.tb03733.x. Ann N Y Acad Sci. 2001. PMID: 11710476 Review.
Cited by
-
Avian auditory hair cell regeneration is accompanied by JAK/STAT-dependent expression of immune-related genes in supporting cells.Development. 2022 Apr 15;149(8):dev200113. doi: 10.1242/dev.200113. Epub 2022 May 3. Development. 2022. PMID: 35420675 Free PMC article.
-
Pharmacological regeneration of sensory hair cells restores afferent innervation and vestibular function.J Clin Invest. 2024 Sep 24;134(22):e181201. doi: 10.1172/JCI181201. J Clin Invest. 2024. PMID: 39316439 Free PMC article.
-
Anatomical and molecular insights into avian inner ear sensory hair cell regeneration.Dev Biol. 2025 Sep;525:13-25. doi: 10.1016/j.ydbio.2025.05.021. Epub 2025 May 23. Dev Biol. 2025. PMID: 40414451 Review.
-
Modelling inner ear development and disease using pluripotent stem cells - a pathway to new therapeutic strategies.Dis Model Mech. 2022 Nov 1;15(11):dmm049593. doi: 10.1242/dmm.049593. Epub 2022 Nov 4. Dis Model Mech. 2022. PMID: 36331565 Free PMC article. Review.
-
Small-molecule inhibition of Lats kinases may promote Yap-dependent proliferation in postmitotic mammalian tissues.Nat Commun. 2021 May 25;12(1):3100. doi: 10.1038/s41467-021-23395-3. Nat Commun. 2021. PMID: 34035288 Free PMC article.
References
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 1995;57:289–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

