Long-term, dynamic synaptic reorganization after GABAergic precursor cell transplantation into adult mouse spinal cord
- PMID: 29134656
- PMCID: PMC6263951
- DOI: 10.1002/cne.24346
Long-term, dynamic synaptic reorganization after GABAergic precursor cell transplantation into adult mouse spinal cord
Abstract
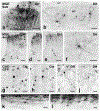
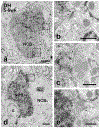
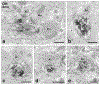
Transplanting embryonic precursors of GABAergic neurons from the medial ganglionic eminence (MGE) into adult mouse spinal cord ameliorates mechanical and thermal hypersensitivity in peripheral nerve injury models of neuropathic pain. Although Fos and transneuronal tracing studies strongly suggest that integration of MGE-derived neurons into host spinal cord circuits underlies recovery of function, the extent to which there is synaptic integration of the transplanted cells has not been established. Here, we used electron microscopic immunocytochemistry to assess directly integration of GFP-expressing MGE-derived neuronal precursors into dorsal horn circuitry in intact, adult mice with short- (5-6 weeks) or long-term (4-6 months) transplants. We detected GFP with pre-embedding avidin-biotin-peroxidase and GABA with post-embedding immunogold labeling. At short and long times post-transplant, we found host-derived synapses on GFP-immunoreactive MGE cells bodies and dendrites. The proportion of dendrites with synaptic input increased from 50% to 80% by 6 months. In all mice, MGE-derived terminals formed synapses with GFP-negative (host) cell bodies and dendrites and, unexpectedly, with some GFP-positive (i.e., MGE-derived) dendrites, possibly reflecting autoapses or cross talk among transplanted neurons. We also observed axoaxonic appositions between MGE and host terminals. Immunogold labeling for GABA confirmed that the transplanted cells were GABAergic and that some transplanted cells received an inhibitory GABAergic input. We conclude that transplanted MGE neurons retain their GABAergic phenotype and integrate dynamically into host-transplant synaptic circuits. Taken together with our previous electrophysiological analyses, we conclude that MGE cells are not GABA pumps, but alleviate pain and itch through synaptic release of GABA.
Keywords: GABA; RRID:AB_476667; RRID:SCR_003577; RRID:SCR_003970; RRID:SCR_007418; RRID:SCR_008487; spinal cord; structural plasticity; transplants; ultrastructure.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Figures







References
-
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, … Alvarez-Buylla A (2009). Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proceedings of the National Academy of Sciences of the United States of America, 106(36), 15472–15477. https://doi.org/10.1073/pnas.0900141106 - DOI - PMC - PubMed
-
- Basbaum AI, Glazer EJ, & Oertel W (1986). Immunoreactive glutamic acid decarboxylase in the trigeminal nucleus caudalis of the cat: A light- and electron-microscopic analysis. Somatosensory Research, 4(1), 77–94. - PubMed
-
- Blizzard CA, Chuckowree JA, King AE, Hosie KA, McCormack GH, Chapman JA, … Dickson TC (2011). Focal damage to the adult rat neocortex induces wound healing accompanied by axonal sprouting and dendritic structural plasticity. Cerebral Cortex, 21(2), 281–291. https://doi.org/10.1093/cercor/bhq091 - DOI - PubMed
-
- Braz J, Solorzano C, Wang X, & Basbaum AI (2014). Transmitting pain and itch messages: A contemporary view of the spinal cord circuits that generate gate control. Neuron, 82(3), 522–536. https://doi.org/10.1016/j.neuron.2014.01.018 - DOI - PMC - PubMed
-
- Braz JM, Juarez-Salinas D, Ross SE, & Basbaum AI (2014). Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. Journal of Clinical Investigation, 124(8), 3612–3616. https://doi.org/10.1172/JCI75214 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

