Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury
- PMID: 29134703
- PMCID: PMC5765410
- DOI: 10.1002/glia.23256
Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury
Abstract
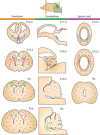
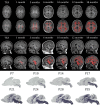
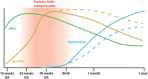
Infants born prematurely are at high risk to develop white matter injury (WMI), due to exposure to hypoxic and/or inflammatory insults. Such perinatal insults negatively impact the maturation of oligodendrocytes (OLs), thereby causing deficits in myelination. To elucidate the precise pathophysiology underlying perinatal WMI, it is essential to fully understand the cellular mechanisms contributing to healthy/normal white matter development. OLs are responsible for myelination of axons. During brain development, OLs are generally derived from neuroepithelial zones, where neural stem cells committed to the OL lineage differentiate into OL precursor cells (OPCs). OPCs, in turn, develop into premyelinating OLs and finally mature into myelinating OLs. Recent studies revealed that OPCs develop in multiple waves and form potentially heterogeneous populations. Furthermore, it has been shown that myelination is a dynamic and plastic process with an excess of OPCs being generated and then abolished if not integrated into neural circuits. Myelination patterns between rodents and humans show high spatial and temporal similarity. Therefore, experimental studies on OL biology may provide novel insights into the pathophysiology of WMI in the preterm infant and offers new perspectives on potential treatments for these patients.
Keywords: brain development; myelination; oligodendrocyte precursor cells; preterm birth; white matter injury.
© 2017 The Authors GLIA Published by Wiley Periodicals, Inc.
Figures


References
-
- Alexandrou, G. , Martensson, G. , Skiold, B. , Blennow, M. , Aden, U. , & Vollmer, B. (2014). White matter microstructure is influenced by extremely preterm birth and neonatal respiratory factors. Acta Paediatrics, 103(1), 48–56. https://doi.org/10.1111/apa.12445 - DOI - PubMed
-
- Ancel, P. Y. , Goffinet, F. , Epipage, 2., Writing Group, Kuhn, P. , Langer, B. , Matis, J. , … Kaminski, M. (2015). Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011: Results of the EPIPAGE‐2 cohort study. JAMA Pediatrics, 169(3), 230–238. https://doi.org/10.1001/jamapediatrics.2014.3351 - DOI - PubMed
-
- Aubert‐Broche, B. , Fonov, V. , Leppert, I. , Pike, G. B. , & Collins, D. L. (2008). Human brain myelination from birth to 4.5 years. Medical Image Computing and Computer‐Assisted Intervention, 11(Pt 2), 180–187. - PubMed
-
- Back, S. A. (2006). Perinatal white matter injury: The changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Mental Retardation and Developmental Disabilities Research Reviews, 12(2), 129–140. https://doi.org/10.1002/mrdd.20107 - DOI - PubMed
-
- Back, S. A. (2017). White matter injury in the preterm infant: Pathology and mechanisms. Acta Neuropathology, https://doi.org/10.1007/s00401-017-1718-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

