Hierarchical Self-Assembly of a Copolymer-Stabilized Coacervate Protocell
- PMID: 29134798
- PMCID: PMC5724030
- DOI: 10.1021/jacs.7b10846
Hierarchical Self-Assembly of a Copolymer-Stabilized Coacervate Protocell
Abstract
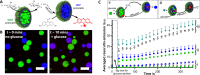
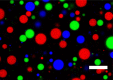
Complex coacervate microdroplets are finding increased utility in synthetic cell applications due to their cytomimetic properties. However, their intrinsic membrane-free nature results in instability that limits their application in protocell research. Herein, we present the development of a new protocell model through the spontaneous interfacial self-assembly of copolymer molecules on biopolymer coacervate microdroplets. This hierarchical protocell model not only incorporates the favorable properties of coacervates (such as spontaneous assembly and macromolecular condensation) but also assimilates the essential features of a semipermeable copolymeric membrane (such as discretization and stabilization). This was accomplished by engineering an asymmetric, biodegradable triblock copolymer molecule comprising hydrophilic, hydrophobic, and polyanionic components capable of direct coacervate membranization via electrostatic surface anchoring and chain self-association. The resulting hierarchical protocell demonstrated striking integrity as a result of membrane formation, successfully stabilizing enzymatic cargo against coalescence and fusion in discrete protocellular populations. The semipermeable nature of the copolymeric membrane enabled the incorporation of a simple enzymatic cascade, demonstrating chemical communication between discrete populations of neighboring protocells. In this way, we pave the way for the development of new synthetic cell constructs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Li M.; Huang X.; Tang T. Y. D.; Mann S. Curr. Opin. Chem. Biol. 2014, 22, 1–11. 10.1016/j.cbpa.2014.05.018. - DOI - PubMed
- Brea R. J.; Hardy M. D.; Devaraj N. K. Chem. - Eur. J. 2015, 21, 12564–12570. 10.1002/chem.201501229. - DOI - PMC - PubMed
- Balasubramanian V.; Herranz-Blanco B.; Almeida P. V.; Hirvonen J.; Santos H. A. Prog. Polym. Sci. 2016, 60, 51–85. 10.1016/j.progpolymsci.2016.04.004. - DOI
- Trantidou T.; Friddin M.; Elani Y.; Brooks N. J.; Law R. V.; Seddon J. M.; Ces O. ACS Nano 2017, 11, 6549–6565. 10.1021/acsnano.7b03245. - DOI - PubMed
- Buddingh’ B. C.; van Hest J. C. M. Acc. Chem. Res. 2017, 50, 769–777. 10.1021/acs.accounts.6b00512. - DOI - PMC - PubMed
-
- Crosby J.; Treadwell T.; Hammerton M.; et al. Chem. Commun. 2012, 48, 11832–11834. 10.1039/c2cc36533b. - DOI - PubMed
- Sokolova E.; Spruijt E.; Hansen M. M. K.; Dubuc E.; Groen J.; Chokkalingam V.; Piruska A.; Heus H. a; Huck W. T. S. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 11692–11697. 10.1073/pnas.1222321110. - DOI - PMC - PubMed
- Elani Y.; Law R. V.; Ces O. Nat. Commun. 2014, 5, 5305. 10.1038/ncomms6305. - DOI - PubMed
- Ichihashi N.; Yomo T. Curr. Opin. Chem. Biol. 2014, 22, 12–17. 10.1016/j.cbpa.2014.06.011. - DOI - PubMed
- Tang T.-Y. D.; van Swaay D.; deMello A.; Ross Anderson J. L.; Mann S. Chem. Commun. 2015, 51, 11429–11432. 10.1039/C5CC04220H. - DOI - PubMed
- Lentini R.; Yeh Martín N.; Mansy S. S. Curr. Opin. Chem. Biol. 2016, 34, 53–61. 10.1016/j.cbpa.2016.06.013. - DOI - PubMed
-
- de Hoog H.-P. M.; Nallani M.; Tomczak N. Soft Matter 2012, 8, 4552. 10.1039/c2sm06934b. - DOI
- Williams D. S.; Patil A. J.; Mann S. Small 2014, 10, 1830–1840. 10.1002/smll.201303654. - DOI - PubMed
- Dewey D. C.; Strulson C. A.; Cacace D. N.; Bevilacqua P. C.; Keating C. D. Nat. Commun. 2014, 5, 4670. 10.1038/ncomms5670. - DOI - PubMed
- Peters R. J. R. W.; Marguet M.; Marais S.; Fraaije M. W.; van Hest J. C. M.; Lecommandoux S. Angew. Chem., Int. Ed. 2014, 53, 146–150. 10.1002/anie.201308141. - DOI - PubMed
- Dora Tang T.-Y.; Rohaida Che Hak C.; Thompson A. J.; Kuimova M. K.; Williams D. S.; Perriman A. W.; Mann S. Nat. Chem. 2014, 6, 527–533. 10.1038/nchem.1921. - DOI - PubMed
- Baxani D. K.; Morgan A. J. L.; Jamieson W. D.; Allender C. J.; Barrow D. A.; Castell O. K. Angew. Chem., Int. Ed. 2016, 55, 14240–14245. 10.1002/anie.201607571. - DOI - PMC - PubMed
- Deng N.-N.; Yelleswarapu M.; Huck W. T. S. J. Am. Chem. Soc. 2016, 138, 7584–7591. 10.1021/jacs.6b02107. - DOI - PubMed
- Mason A. F.; Thordarson P. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 3817–3825. 10.1002/pola.28780. - DOI
- Mytnyk S.; Olive A. G. L.; Versluis F.; Poolman J. M.; Mendes E.; Eelkema R.; van Esch J. H. Angew. Chem., Int. Ed. 2017, 56, 1–7. 10.1002/anie.201706272. - DOI - PubMed
-
- Xu J.; Sigworth F. J.; LaVan D. A. Adv. Mater. 2010, 22, 120–127. 10.1002/adma.200901945. - DOI - PMC - PubMed
- Grzybowski B. A.; Huck W. T. S. Nat. Nanotechnol. 2016, 11, 585–592. 10.1038/nnano.2016.116. - DOI - PubMed
- Rodríguez-Arco L.; Li M.; Mann S. Nat. Mater. 2017, 16, 857–863. 10.1038/nmat4916. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

