Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes
- PMID: 29134959
- PMCID: PMC7938338
- DOI: 10.1038/nrclinonc.2017.175
Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes
Erratum in
-
Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes.Nat Rev Clin Oncol. 2018 Mar;15(3):150. doi: 10.1038/nrclinonc.2017.188. Epub 2017 Nov 28. Nat Rev Clin Oncol. 2018. PMID: 29182164
Abstract
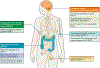
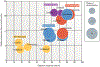
The gene encoding the receptor-tyrosine kinase RET was first discovered more than three decades ago, and activating RET rearrangements and mutations have since been identified as actionable drivers of oncogenesis. Several multikinase inhibitors with activity against RET have been explored in the clinic, and confirmed responses to targeted therapy with these agents have been observed in patients with RET-rearranged lung cancers or RET-mutant thyroid cancers. Nevertheless, response rates to RET-directed therapy are modest compared with those achieved using targeted therapies matched to other oncogenic drivers of solid tumours, such as sensitizing EGFR or BRAFV600E mutations, or ALK or ROS1 rearrangements. To date, no RET-directed targeted therapeutic has received regulatory approval for the treatment of molecularly defined populations of patients with RET-mutant or RET-rearranged solid tumours. In this Review, we discuss how emerging data have informed the debate over whether the limited success of multikinase inhibitors with activity against RET can be attributed to the tractability of RET as a drug target or to the lack, until 2017, of highly specific inhibitors of this oncoprotein in the clinic. We emphasize that novel approaches to targeting RET-dependent tumours are necessary to improve the clinical efficacy of single-agent multikinase inhibition and, thus, hasten approvals of RET-directed targeted therapies.
Conflict of interest statement
Competing interests statement
A.D. has received honoraria from and has been an advisory board member for Ariad, AstraZeneca, Blueprint Medicines, Exelixis, Genentech/Roche, Ignyta, and Loxo Oncology; he has received honoraria from Foundation Medicine. The other authors declare no competing interests.
Figures



References
-
- Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med 344, 1031–1037 (2001). - PubMed
-
- Mok TS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med 361, 947–957 (2009). - PubMed
-
- Solomon BJ, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med 371, 2167–2177 (2014). - PubMed
-
- Planchard D, et al. Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 18, 1307–1316 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

