Implementation and performance of the artificial force induced reaction method in the GRRM17 program
- PMID: 29135034
- PMCID: PMC5765425
- DOI: 10.1002/jcc.25106
Implementation and performance of the artificial force induced reaction method in the GRRM17 program
Abstract
This article reports implementation and performance of the artificial force induced reaction (AFIR) method in the upcoming 2017 version of GRRM program (GRRM17). The AFIR method, which is one of automated reaction path search methods, induces geometrical deformations in a system by pushing or pulling fragments defined in the system by an artificial force. In GRRM17, three different algorithms, that is, multicomponent algorithm (MC-AFIR), single-component algorithm (SC-AFIR), and double-sphere algorithm (DS-AFIR), are available, where the MC-AFIR was the only algorithm which has been available in the previous 2014 version. The MC-AFIR does automated sampling of reaction pathways between two or more reactant molecules. The SC-AFIR performs automated generation of global or semiglobal reaction path network. The DS-AFIR finds a single path between given two structures. Exploration of minimum energy structures within the hypersurface in which two different electronic states degenerate, and an interface with the quantum mechanics/molecular mechanics method, are also described. A code termed SAFIRE will also be available, as a visualization software for complicated reaction path networks. © 2017 The Authors. Journal of Computational Chemistry Published by Wiley Periodicals, Inc.
Keywords: QM/MM; intrinsic reaction coordinate; nonadiabatic transition; potential energy surface; transition state.
© 2017 The Authors. Journal of Computational Chemistry Published by Wiley Periodicals, Inc.
Figures





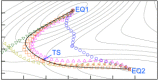

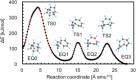


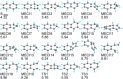
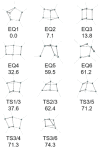
References
-
- Schlegel H. B., J. Comput. Chem. 2003, 24, 1514. - PubMed
-
- Jensen F., Introduction to Computational Chemistry, 2nd ed.; Wiley: Chichester, UK, 2007.
-
- Fukui K., Acc. Chem. Res. 1981, 14, 363.
-
- Maeda S., Harabuchi Y., Ono Y., Taketsugu T., Morokuma K., Int. J. Quantum Chem. 2015, 115, 258.
-
- Harvey J. N., WIREs Comput. Mol. Sci. 2014, 4, 1.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

