Cerebral artery myogenic reactivity: The next frontier in developing effective interventions for subarachnoid hemorrhage
- PMID: 29135346
- PMCID: PMC5757446
- DOI: 10.1177/0271678X17742548
Cerebral artery myogenic reactivity: The next frontier in developing effective interventions for subarachnoid hemorrhage
Abstract
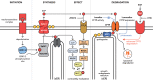
Aneurysmal subarachnoid hemorrhage (SAH) is a devastating cerebral event that kills or debilitates the majority of those afflicted. The blood that spills into the subarachnoid space stimulates profound cerebral artery vasoconstriction and consequently, cerebral ischemia. Thus, once the initial bleeding in SAH is appropriately managed, the clinical focus shifts to maintaining/improving cerebral perfusion. However, current therapeutic interventions largely fail to improve clinical outcome, because they do not effectively restore normal cerebral artery function. This review discusses emerging evidence that perturbed cerebrovascular "myogenic reactivity," a crucial microvascular process that potently dictates cerebral perfusion, is the critical element underlying cerebral ischemia in SAH. In fact, the myogenic mechanism could be the reason why many therapeutic interventions, including "Triple H" therapy, fail to deliver benefit to patients. Understanding the molecular basis for myogenic reactivity changes in SAH holds the key to develop more effective therapeutic interventions; indeed, promising recent advancements fuel optimism that vascular dysfunction in SAH can be corrected to improve outcome.
Keywords: Cerebral blood flow; cystic fibrosis transmembrane conductance regulator (CFTR); microvascular dysfunction; myogenic vasoconstriction; tumor necrosis factor.
Figures

References
-
- Tso MK, Macdonald RL. Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl Stroke Res 2014; 5: 174–189. - PubMed
-
- Solenski NJ, Haley EC, Kassell NF, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med 1995; 23: 1007–1017. - PubMed
-
- Hop JW, Rinkel GJ, Algra A, et al. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 1997; 28: 660–664. - PubMed
-
- Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998; 50: 1413–1418. - PubMed
-
- van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007; 369: 306–318. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

