Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE-/- Mice
- PMID: 29135917
- PMCID: PMC5706047
- DOI: 10.3390/md15110358
Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE-/- Mice
Abstract
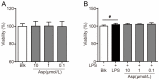
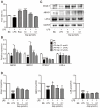
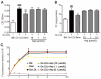
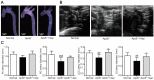
Asperlin is a marine-derived natural product with antifungal and anti-inflammatory activities in vitro. In the present study, we isolated asperlin from a marine Aspergillus versicolor LZD4403 fungus and investigated its anti-atherosclerotic effects in vitro and in vivo. Asperlin significantly inhibited lipopolysaccharides (LPS)- but not oxidated low-density lipoprotein (oxLDL)-evoked foam cell formation and promoted cholesterol efflux in RAW264.7 macrophages. Supplementation with asperlin also suppressed LPS-elicited production of pro-inflammatory factors in RAW264.7 macrophages, decreased the expression levels of iNOS, IL-1β and TNFα, and increased the expression of IL-10 and IL-4, indicating a remarkable shift in M1/M2 macrophages polarization. In vivo experiments in high-fat diet (HFD)-fed ApoE-/- mice showed that oral administration of asperlin for 12 weeks remarkably suppressed atherosclerotic plaque formation in the aorta, as revealed by the reduced aortic dilatation and decreased atherosclerotic lesion area. Asperlin also decreased serum levels of pro-inflammatory factors but showed little impact on blood lipids in ApoE-/- atherosclerotic mice. These results suggested that asperlin is adequate to prevent atherosclerosis in vivo. It may exert atheroprotective function through suppressing inflammation rather than ameliorating dyslipidemia.
Keywords: M1/M2 polarization; asperlin; atherosclerosis; foam cell; inflammation; macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
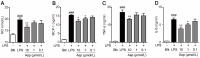
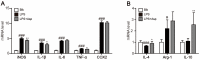
Similar articles
-
Asperlin from the marine-derived fungus Aspergillus sp. SF-5044 exerts anti-inflammatory effects through heme oxygenase-1 expression in murine macrophages.J Pharmacol Sci. 2011;116(3):283-95. doi: 10.1254/jphs.10219fp. Epub 2011 Jun 25. J Pharmacol Sci. 2011. PMID: 21705844
-
Retinoic acid induces macrophage cholesterol efflux and inhibits atherosclerotic plaque formation in apoE-deficient mice.Br J Nutr. 2015 Aug 28;114(4):509-18. doi: 10.1017/S0007114515002159. Epub 2015 Jul 23. Br J Nutr. 2015. PMID: 26201974
-
Curcuma oil attenuates accelerated atherosclerosis and macrophage foam-cell formation by modulating genes involved in plaque stability, lipid homeostasis and inflammation.Br J Nutr. 2015 Jan 14;113(1):100-13. doi: 10.1017/S0007114514003195. Epub 2014 Nov 13. Br J Nutr. 2015. PMID: 25391643
-
Anti-atherogenic and anti-inflammatory properties of glucagon-like peptide-1, glucose-dependent insulinotropic polypepide, and dipeptidyl peptidase-4 inhibitors in experimental animals.J Diabetes Investig. 2016 Apr;7 Suppl 1(Suppl 1):80-6. doi: 10.1111/jdi.12446. Epub 2016 Mar 31. J Diabetes Investig. 2016. PMID: 27186361 Free PMC article. Review.
-
Targeting foam cell formation and macrophage polarization in atherosclerosis: The Therapeutic potential of rhubarb.Biomed Pharmacother. 2020 Sep;129:110433. doi: 10.1016/j.biopha.2020.110433. Epub 2020 Jun 28. Biomed Pharmacother. 2020. PMID: 32768936 Review.
Cited by
-
Isolation and Identification of Isocoumarin Derivatives With Specific Inhibitory Activity Against Wnt Pathway and Metabolome Characterization of Lasiodiplodia venezuelensis.Front Chem. 2021 Aug 12;9:664489. doi: 10.3389/fchem.2021.664489. eCollection 2021. Front Chem. 2021. PMID: 34458231 Free PMC article.
-
Desulfovibrio desulfuricans aggravates atherosclerosis by enhancing intestinal permeability and endothelial TLR4/NF-κB pathway in Apoe -/- mice.Genes Dis. 2021 Oct 16;10(1):239-253. doi: 10.1016/j.gendis.2021.09.007. eCollection 2023 Jan. Genes Dis. 2021. PMID: 37013030 Free PMC article.
-
Marine Fungi Bioactives with Anti-Inflammatory, Antithrombotic and Antioxidant Health-Promoting Properties Against Inflammation-Related Chronic Diseases.Mar Drugs. 2024 Nov 18;22(11):520. doi: 10.3390/md22110520. Mar Drugs. 2024. PMID: 39590800 Free PMC article. Review.
-
Marine Natural Products and Coronary Artery Disease.Front Cardiovasc Med. 2021 Sep 21;8:739932. doi: 10.3389/fcvm.2021.739932. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34621803 Free PMC article. Review.
-
Marine-Derived Bioactive Ingredients in Functional Foods for Aging: Nutritional and Therapeutic Perspectives.Mar Drugs. 2024 Nov 4;22(11):496. doi: 10.3390/md22110496. Mar Drugs. 2024. PMID: 39590776 Free PMC article. Review.
References
-
- Li A.C., Binder C.J., Gutierrez A., Brown K.K., Plotkin C.R., Pattison J.W., Valledor A.F., Davis R.A., Willson T.M., Witztum J.L., et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J. Clin. Investig. 2004;114:1564–1576. doi: 10.1172/JCI18730. - DOI - PMC - PubMed
-
- Chawla A., Boisvert W.A., Lee C.H., Laffitte B.A., Barak Y., Joseph S.B., Liao D., Nagy L., Edwards P.A., Curtiss L.K., et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 2001;7:161–171. doi: 10.1016/S1097-2765(01)00164-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

