Nox, Reactive Oxygen Species and Regulation of Vascular Cell Fate
- PMID: 29135921
- PMCID: PMC5745500
- DOI: 10.3390/antiox6040090
Nox, Reactive Oxygen Species and Regulation of Vascular Cell Fate
Abstract
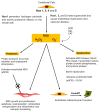
The generation of reactive oxygen species (ROS) and an imbalance of antioxidant defence mechanisms can result in oxidative stress. Several pro-atherogenic stimuli that promote intimal-medial thickening (IMT) and early arteriosclerotic disease progression share oxidative stress as a common regulatory pathway dictating vascular cell fate. The major source of ROS generated within the vascular system is the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family of enzymes (Nox), of which seven members have been characterized. The Nox family are critical determinants of the redox state within the vessel wall that dictate, in part the pathophysiology of several vascular phenotypes. This review highlights the putative role of ROS in controlling vascular fate by promoting endothelial dysfunction, altering vascular smooth muscle phenotype and dictating resident vascular stem cell fate, all of which contribute to intimal medial thickening and vascular disease progression.
Keywords: Nox; ROS; adventitial cells; arteriosclerotic disease; endothelial; intimal-medial thickening; stem cells; vascular smooth muscle.
Conflict of interest statement
The authors declare no conflicts of interest
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

