Adolescent Alcohol Drinking Renders Adult Drinking BLA-Dependent: BLA Hyper-Activity as Contributor to Comorbid Alcohol Use Disorder and Anxiety Disorders
- PMID: 29135933
- PMCID: PMC5704158
- DOI: 10.3390/brainsci7110151
Adolescent Alcohol Drinking Renders Adult Drinking BLA-Dependent: BLA Hyper-Activity as Contributor to Comorbid Alcohol Use Disorder and Anxiety Disorders
Abstract
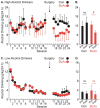
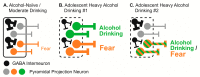
Adolescent alcohol drinking increases the risk for alcohol-use disorder in adulthood. Yet, the changes in adult neural function resulting from adolescent alcohol drinking remain poorly understood. We hypothesized that adolescent alcohol drinking alters basolateral amygdala (BLA) function, making alcohol drinking BLA-dependent in adulthood. Male, Long Evans rats were given voluntary, intermittent access to alcohol (20% ethanol) or a bitter, isocaloric control solution, across adolescence. Half of the rats in each group received neurotoxic BLA lesions. In adulthood, all rats were given voluntary, intermittent access to alcohol. BLA lesions reduced adult alcohol drinking in rats receiving adolescent access to alcohol, but not in rats receiving adolescent access to the control solution. The effect of the BLA lesion was most apparent in high alcohol drinking adolescent rats. The BLA is essential for fear learning and is hyper-active in anxiety disorders. The results are consistent with adolescent heavy alcohol drinking inducing BLA hyper-activity, providing a neural mechanism for comorbid alcohol use disorder and anxiety disorders.
Keywords: amygdala; chronic; ethanol; fear; intermittent; rat; voluntary.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Substance Abuse and Mental Health Services Administration . In: Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville M.D., editor. Department of Health and Human Services; Washington, DC, USA: 2014. (Volume NSDUH Series H-48).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

