Arginase expression modulates nitric oxide production in Leishmania (Leishmania) amazonensis
- PMID: 29135983
- PMCID: PMC5685479
- DOI: 10.1371/journal.pone.0187186
Arginase expression modulates nitric oxide production in Leishmania (Leishmania) amazonensis
Abstract
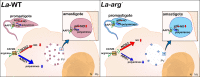
Background: Arginase is an enzyme that converts L-arginine to urea and L-ornithine, an essential substrate for the polyamine pathway supporting Leishmania (Leishmania) amazonensis replication and its survival in the mammalian host. L-arginine is also the substrate of macrophage nitric oxide synthase 2 (NOS2) to produce nitric oxide (NO) that kills the parasite. This competition can define the fate of Leishmania infection.
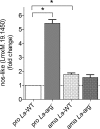
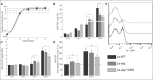
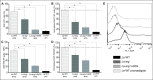
Methodology/principal findings: The transcriptomic profiling identified a family of oxidoreductases in L. (L.) amazonensis wild-type (La-WT) and L. (L.) amazonensis arginase knockout (La-arg-) promastigotes and axenic amastigotes. We highlighted the identification of an oxidoreductase that could act as nitric oxide synthase-like (NOS-like), due to the following evidences: conserved domain composition, the participation of NO production during the time course of promastigotes growth and during the axenic amastigotes differentiation, regulation dependence on arginase activity, as well as reduction of NO amount through the NOS activity inhibition. NO quantification was measured by DAF-FM labeling analysis in a flow cytometry.
Conclusions/significance: We described an arginase-dependent NOS-like activity in L. (L.) amazonensis and its role in the parasite growth. The increased detection of NO production in the mid-stationary and late-stationary growth phases of La-WT promastigotes could suggest that this production is an important factor to metacyclogenesis triggering. On the other hand, La-arg- showed an earlier increase in NO production compared to La-WT, suggesting that NO production can be arginase-dependent. Interestingly, La-WT and La-arg- axenic amastigotes produced higher levels of NO than those observed in promastigotes. As a conclusion, our work suggested that NOS-like is expressed in Leishmania in the stationary growth phase promastigotes and amastigotes, and could be correlated to metacyclogenesis and amastigotes growth in a dependent way to the internal pool of L-arginine and arginase activity.
Conflict of interest statement
Figures



References
-
- Bogdan C (2008) Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol 10: 1221–1234. doi: 10.1111/j.1462-5822.2008.01146.x - DOI - PubMed
-
- Liese J, Schleicher U, Bogdan C (2008) The innate immune response against Leishmania parasites. Immunobiology 213: 377–387. doi: 10.1016/j.imbio.2007.12.005 - DOI - PubMed
-
- Sacks D, Kamhawi S (2001) Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol 55: 453–483. doi: 10.1146/annurev.micro.55.1.453 - DOI - PubMed
-
- Teixeira DE, Benchimol M, Rodrigues JC, Crepaldi PH, Pimenta PF, de Souza W (2013) The cell biology of Leishmania: how to teach using animations. PLoS Pathog 9: e1003594 doi: 10.1371/journal.ppat.1003594 - DOI - PMC - PubMed
-
- Séguin O, Descoteaux A (2016) Leishmania, the phagosome, and host responses: The journey of a parasite. Cell Immunol 309: 1–6. doi: 10.1016/j.cellimm.2016.08.004 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

