Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- PMID: 29136032
- PMCID: PMC5685482
- DOI: 10.1371/journal.pmed.1002432
Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
Abstract
Background: Although WHO recommends viral load (VL) monitoring for those on antiretroviral therapy (ART), availability in low-income countries remains limited. We investigated long-term VL and resistance in HIV-infected children managed without real-time VL monitoring.
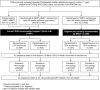
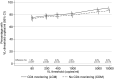
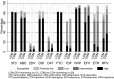
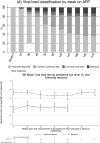
Methods and findings: In the ARROW factorial trial, 1,206 children initiating ART in Uganda and Zimbabwe between 15 March 2007 and 18 November 2008, aged a median 6 years old, with median CD4% of 12%, were randomised to monitoring with or without 12-weekly CD4 counts and to receive 2 nucleoside reverse transcriptase inhibitors (2NRTI, mainly abacavir+lamivudine) with a non-nucleoside reverse transcriptase inhibitor (NNRTI) or 3 NRTIs as long-term ART. All children had VL assayed retrospectively after a median of 4 years on ART; those with >1,000 copies/ml were genotyped. Three hundred and sixteen children had VL and genotypes assayed longitudinally (at least every 24 weeks). Overall, 67 (6%) switched to second-line ART and 54 (4%) died. In children randomised to WHO-recommended 2NRTI+NNRTI long-term ART, 308/378 (81%) monitored with CD4 counts versus 297/375 (79%) without had VL <1,000 copies/ml at 4 years (difference = +2.3% [95% CI -3.4% to +8.0%]; P = 0.43), with no evidence of differences in intermediate/high-level resistance to 11 drugs. Among children with longitudinal VLs, only 5% of child-time post-week 24 was spent with persistent low-level viraemia (80-5,000 copies/ml) and 10% with VL rebound ≥5,000 copies/ml. No child resuppressed <80 copies/ml after confirmed VL rebound ≥5,000 copies/ml. A median of 1.0 (IQR 0.0,1.5) additional NRTI mutation accumulated over 2 years' rebound. Nineteen out of 48 (40%) VLs 1,000-5,000 copies/ml were immediately followed by resuppression <1,000 copies/ml, but only 17/155 (11%) VLs ≥5,000 copies/ml resuppressed (P < 0.0001). Main study limitations are that analyses were exploratory and treatment initiation used 2006 criteria, without pre-ART genotypes.
Conclusions: In this study, children receiving first-line ART in sub-Saharan Africa without real-time VL monitoring had good virological and resistance outcomes over 4 years, regardless of CD4 monitoring strategy. Many children with detectable low-level viraemia spontaneously resuppressed, highlighting the importance of confirming virological failure before switching to second-line therapy. Children experiencing rebound ≥5,000 copies/ml were much less likely to resuppress, but NRTI resistance increased only slowly. These results are relevant to the increasing numbers of HIV-infected children receiving first-line ART in sub-Saharan Africa with limited access to virological monitoring.
Trial registration: ISRCTN Registry, ISRCTN24791884.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: AJP has received funding from the Wellcome Trust as a personal Fellowship grant (108065/Z/15/Z); has received payment from the PENTA Foundation to provide online teaching materials in paediatric HIV; and was an invited speaker at a meeting unrelated to the submitted work in South Africa in April 2014 (81st Nestle Nutrition Institute Workshop Program). The institution of DMG (MRC Clinical Trials Unit at UCL) receives funding for her participation on the ViiV cabotegravir advisory board; and as grants from the EU, MRC UK, Wellcome Trust, and Department of International Development (DFID). ViiV, Gilead, Merck, Cipla, and Mylan have donated drugs for trials supported by this funding. DMG is a current member of the Joint Global Health Trials panel for MRC, Wellcome Trust, and DFID; has reviewed grants for EU and chaired EDCTP grant review board, for which DMG receives no direct funding. The institution of ASW (MRC Clinical Trials Unit at UCL) has received funding for her participation in Data Safety Monitoring Boards (Janssen) and lecturing (Gilead Sciences).
Figures
References
-
- Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: 2014.
-
- Joint United Nations Programme on HIV/AIDS. AIDS by the numbers. 2016. http://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-number....
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. 2016. http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf. - PubMed
-
- Joint United Nations Programme on HIV/AIDS. Diagnostics access initiative to achieve the 90-90-90 treatment target. 2015. http://www.unaids.org/sites/default/files/media_asset/20150422_diagnosti....
-
- Sigaloff KCE, Calis JCJ, Geelen SP, van Vugt M, de Wit TFR. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis. 2011;11:769–79. doi: 10.1016/S1473-3099(11)70141-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

