Comparative efficacy and safety of antibiotics used to treat acute bacterial skin and skin structure infections: Results of a network meta-analysis
- PMID: 29136035
- PMCID: PMC5685605
- DOI: 10.1371/journal.pone.0187792
Comparative efficacy and safety of antibiotics used to treat acute bacterial skin and skin structure infections: Results of a network meta-analysis
Abstract
Objective: This NMA compared the efficacy and safety between IV antibiotics that are used in the current standard of care for managing adult patients (≥18 years of age) with ABSSSI.
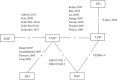
Methods: Comparators were chosen on the basis that both direct and indirect comparisons between the interventions of interest could be performed. Outcomes of the analysis were selected on the basis that they are frequently measured and reported in trials involving ABSSSI patients, and only published randomised control trials of any size and duration and with any blinding status were eligible for inclusion in the analysis. The NMA was performed using both a fixed-effect and random-effect model. Efficacy-related endpoints were (1) clinical treatment success and (2) microbiological success at TOC visit. Safety-related endpoints were (1) number of discontinuations due to AEs/SAEs, (2) patients experiencing AEs, (3) patients experiencing SAEs and (4) all-cause mortality.
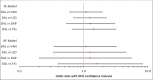
Results: Study interventions included daptomycin, dalbavancin, linezolid and tigecycline. Vancomycin was the comparator in all studies, except in two where it was linezolid and teicoplanin. The NMA showed that irrespective of patient subgroup, the likelihood of clinical and microbiological success with dalbavancin was statistically similar to the comparators studied. No statistically significant differences were observed between dalbavancin and any of the comparators in the discontinuation rate due to AEs/SAEs. In contrast, dalbavancin was associated with a significantly lower likelihood of experiencing an AE than linezolid, a significantly lower likelihood of experiencing a SAE than vancomycin and daptomycin, and a significantly lower risk of all-cause mortality than vancomycin, linezolid and tigecycline.
Conclusion: Dalbavancin affords a promising, new alternative IV antimicrobial agent which is as effective as traditional therapies, but with the added benefit of enabling clinicians to treat patients with ABSSSI in different organisational settings. Notwithstanding, any introduction of an effective treatment with a differential mode of administration into healthcare systems must be followed by a change in clinical practice and patient management in order to fully achieve desirable economic outcomes.
Conflict of interest statement
Figures

References
-
- Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis. 2013;13:252 doi: 10.1186/1471-2334-13-252 ; PubMed Central PMCID: PMCPMC3679727. - DOI - PMC - PubMed
-
- Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168(14):1585–91. doi: 10.1001/archinte.168.14.1585 . - DOI - PubMed
-
- FDA. Guidance for industry acute bacterial skin and skin structure infections: developing drugs for treatment Silver Spring, Maryland, USA: Food and Drug Administration; 2013.
-
- Shor AF. Epidemiology and economic impact of methicillinresistant Staphylococcus aureus: review and analysis of the literature. Pharmacoeconomics. 2007;25:751–68. PubMed Central PMCID: PMC17803334. - PubMed
-
- Logman JF, Stephens J, Heeg B, Haider S, Cappelleri J, Nathwani D, et al. Comparative effectiveness of antibiotics for the treatment of MRSA complicated skin and soft tissue infections. Curr Med Res Opin. 2010;26(7):1565–78. doi: 10.1185/03007995.2010.481251 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

