Contextual Fear Extinction Induces Hippocampal Metaplasticity Mediated by Metabotropic Glutamate Receptor 5
- PMID: 29136107
- PMCID: PMC6454528
- DOI: 10.1093/cercor/bhx282
Contextual Fear Extinction Induces Hippocampal Metaplasticity Mediated by Metabotropic Glutamate Receptor 5
Abstract
Dysregulated fear memory can lead to a broad spectrum of anxiety disorders. The brain systems underlying fear memory are manifold, with the hippocampus being prominently involved by housing fear-related spatial memories as engrams, which are created and stored through neural changes such as synaptic plasticity. Although metabotropic glutamate (mGlu) receptors contribute significantly to both fear behavior and hippocampal synaptic plasticity, the relationship between these two phenomena has not been fully elucidated. Here, we report that contextual fear extinction induces a novel form of metaplasticity mediated by mGlu5 at the hippocampal SC-CA1 synapse. Further, blockade of mGlu5 prevents both contextual fear extinction and expression of this metaplasticity. This form of metaplasticity was absent in a mouse model of MECP2-duplication syndrome, corresponding to a complete deficit in extinction learning. These findings suggest that mGlu5-dependent metaplasticity within the hippocampus may play a critical role in extinction of contextual fear.
Figures
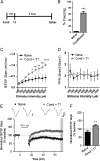
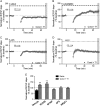
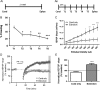
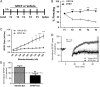



References
-
- Abel T, Lattal KM. 2001. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 11(2):180–187. - PubMed
-
- Abraham WC. 2008. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 9(5):387. - PubMed
-
- Abraham WC, Bear MF. 1996. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19(4):126–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

