A molecular cascade modulates MAP1B and confers resistance to mTOR inhibition in human glioblastoma
- PMID: 29136244
- PMCID: PMC5961175
- DOI: 10.1093/neuonc/nox215
A molecular cascade modulates MAP1B and confers resistance to mTOR inhibition in human glioblastoma
Abstract
Background: Clinical trials of therapies directed against nodes of the signaling axis of phosphatidylinositol-3 kinase/Akt/mammalian target of rapamycin (mTOR) in glioblastoma (GBM) have had disappointing results. Resistance to mTOR inhibitors limits their efficacy.
Methods: To determine mechanisms of resistance to chronic mTOR inhibition, we performed tandem screens on patient-derived GBM cultures.
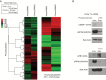
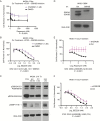
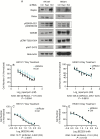
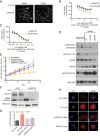
Results: An unbiased phosphoproteomic screen quantified phosphorylation changes associated with chronic exposure to the mTOR inhibitor rapamycin, and our analysis implicated a role for glycogen synthase kinase (GSK)3B attenuation in mediating resistance that was confirmed by functional studies. A targeted short hairpin RNA screen and further functional studies both in vitro and in vivo demonstrated that microtubule-associated protein (MAP)1B, previously associated predominantly with neurons, is a downstream effector of GSK3B-mediated resistance. Furthermore, we provide evidence that chronic rapamycin induces microtubule stability in a MAP1B-dependent manner in GBM cells. Additional experiments explicate a signaling pathway wherein combinatorial extracellular signal-regulated kinase (ERK)/mTOR targeting abrogates inhibitory phosphorylation of GSK3B, leads to phosphorylation of MAP1B, and confers sensitization.
Conclusions: These data portray a compensatory molecular signaling network that imparts resistance to chronic mTOR inhibition in primary, human GBM cell cultures and points toward new therapeutic strategies.
Figures
References
-
- Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. - PubMed
-
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–348. - PubMed
-
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

