Effect of antitumor treatments on triple-negative breast cancer patients: A PRISMA-compliant network meta-analysis of randomized controlled trials
- PMID: 29137021
- PMCID: PMC5690714
- DOI: 10.1097/MD.0000000000008389
Effect of antitumor treatments on triple-negative breast cancer patients: A PRISMA-compliant network meta-analysis of randomized controlled trials
Abstract
Background: Triple-negative breast cancer (TNBC) lacks the expression of the estrogen receptor, progesterone receptor, and receptor tyrosine-protein kinase erbB-2 (HER2/neu), which renders hormone-related endocrine and targeted therapy essentially futile.
Objective: We performed a meta-analysis to assess the effects of antitumor regimens in the treatment of TNBC patients.
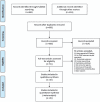
Methods: We searched electronic databases, including PubMed, Embase, and the Cochrane Library, through January 2017 using the following keywords: "triple negative breast cancer," "TNBC," and "random*" without language restrictions. The major outcome in the present analysis was the overall response rate (ORR), and the secondary outcomes were progression-free survival (PFS) and overall survival (OS). A network meta-analysis and multilevel mixed-effects logistic regression were used to compare antitumor regimens.
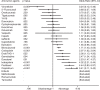
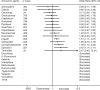
Results: We included 35 articles assessing a total of 8476 TNBC patients in our systematic review. The regimen of Bevacizumab, Carboplatin, and Paclitaxel (78.2%) was the most likely to improve the ORR in TNBC patients, followed by EndoTAG-1 and Paclitaxel (69.7%), Carboplatin and Paclitaxel (65.0%), and Bevacizumab and Paclitaxel (61.8%). In the patients without metastasis, the regimen of Bevacizumab, Carboplatin, and Paclitaxel (74.9%) remained the most likely to improve the ORR. We could not analyze the results for patients with metastasis or outcomes of PFS and OS because no >4 regimens formed a network. In the regression analysis, Bevacizumab (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.43-2.05; P < .001) and Carboplatin (OR, 2.07; 95% CI, 1.62-2.64; P < .001) correlated with superior ORR outcome, and Iniparib (OR, 1.51; 95% CI, 1.11-2.07; P = .009) correlated with superior OS outcome.
Conclusion: The regimen including Bevacizumab, Carboplatin, and Paclitaxel was the most likely to improve the ORR in TNBC patients and in advanced metastatic TNBC patients. The administration of Bevacizumab and Carboplatin provided greater benefit toward improved patient ORR.
Conflict of interest statement
The authors report no conflicts of interest.
Figures



References
-
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. - PubMed
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938–48. - PubMed
-
- Duffy MJ, McGowan PM, Crown J. Targeted therapy for triple-negative breast cancer: where are we? Int J Cancer 2012;131:2471–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

