A multicenter, open-label, phase III study of Abcertin in Gaucher disease
- PMID: 29137040
- PMCID: PMC5690733
- DOI: 10.1097/MD.0000000000008492
A multicenter, open-label, phase III study of Abcertin in Gaucher disease
Erratum in
-
A multicenter, open-label, phase III study of Abcertin in Gaucher disease: Erratum.Medicine (Baltimore). 2018 Aug;97(33):e12066. doi: 10.1097/MD.0000000000012066. Medicine (Baltimore). 2018. PMID: 30113507 Free PMC article. No abstract available.
Abstract
Background: Gaucher disease (GD) is caused by a deficiency in the lysosomal enzyme glucocerebrosidase. Enzyme replacement therapy (ERT) is recommended for clinical improvement.
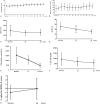
Methods: The efficacy and safety of a new imiglucerase, Abcertin, were assessed in 7 Egyptian patients with treatment-naïve type 1 GD. Each patient was administered a biweekly 60 U/kg dose of Abcertin for 6 months. The primary endpoint was the change in hemoglobin concentration. The secondary endpoints were changes from baseline in platelet counts, spleen and liver volumes, biomarker levels, skeletal parameters, and bone mineral density.
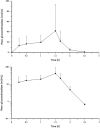
Results: The hemoglobin concentration increased by a mean of 1.96 ± 0.91 g/dL (range 1.11-2.80 g/dL) or 20.6% (P = .001). Statistically significant increases in the platelet count and decreases in the spleen volume and biomarker levels were also observed. There were no severe drug-related adverse events. One patient developed anti-imiglucerase antibodies without neutralizing activity.
Conclusion: Our study results demonstrate the efficacy and safety of Abcertin in patients with type 1 GD. This suggests that Abcertin can be an alternative ERT option for type 1 GD.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Grabowski GA. Recent clinical progress in Gaucher disease. Curr Opin Pediatr 2005;17:519–24. - PubMed
-
- Zimran A, Elstein D, Levy-Lahad E, et al. Replacement therapy with imiglucerase for type 1 Gaucher's disease. Lancet 1995;345:1479–80. - PubMed
-
- Cox TM, Schofield JP. Gaucher's disease: clinical features and natural history. Baillieres Clin Haematol 1997;10:657–89. - PubMed
-
- Kim JW, Liou BB, Lai MY, et al. Gaucher disease: identification of three new mutations in the Korean and Chinese (Taiwanese) populations. Hum Mutat 1996;7:214–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

