NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [67Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice
- PMID: 29137110
- PMCID: PMC6150197
- DOI: 10.3390/molecules22111950
NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [67Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice
Abstract
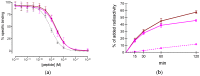
The GRPR-antagonist-based radioligands [67/68Ga/111In/177Lu]NeoBOMB1 have shown excellent theragnostic profiles in preclinical prostate cancer models, while [68Ga]NeoBOMB1 effectively visualized prostate cancer lesions in patients. We were further interested to explore the theragnostic potential of NeoBOMB1 in GRPR-positive mammary carcinoma, by first studying [67Ga]NeoBOMB1 in breast cancer models; Methods: We investigated the profile of [67Ga]NeoBOMB1, a [68Ga]NeoBOMB1 surrogate, in GRPR-expressing T-47D cells and animal models; Results: NeoBOMB1 (IC50s of 2.2 ± 0.2 nM) and [natGa]NeoBOMB1 (IC50s of 2.5 ± 0.2 nM) exhibited high affinity for the GRPR. At 37 °C [67Ga]NeoBOMB1 strongly bound to the T-47D cell-membrane (45.8 ± 0.4% at 2 h), internalizing poorly, as was expected for a radioantagonist. [67Ga]NeoBOMB1 was detected >90% intact in peripheral mouse blood at 30 min pi. In mice bearing T-47D xenografts, [67Ga]NeoBOMB1 specifically localized in the tumor (8.68 ± 2.9% ID/g vs. 0.6 ± 0.1% ID/g during GRPR-blockade at 4 h pi). The unfavorably high pancreatic uptake could be considerably reduced (206.29 ± 17.35% ID/g to 42.46 ± 1.31% ID/g at 4 h pi) by increasing the NeoBOMB1 dose from 10 pmol to 200 pmol, whereas tumor uptake remained unaffected. Notably, tumor values did not decline from 1 to 24 h pi; Conclusions: [67Ga]NeoBOMB1 can successfully target GRPR-positive breast cancer in animals with excellent prospects for clinical translation.
Keywords: GRPR-antagonist; PET-imaging; breast cancer; targeted tumor imaging; theragnostics.
Conflict of interest statement
F.O., D.B. and M.T., are AAA employees. T.M., B.A.N, and M.d.J. are co-inventors of an AAA-patent application (GRPR-Antagonists for detection, diagnosis and treatment of GRPR-positive cancer. WO 2014052471 A1); AAA funded this study and participated in the decision to publish the results. Funds for covering the costs to publish in open access were provided by AAA.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

