Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications
- PMID: 29137195
- PMCID: PMC5706251
- DOI: 10.3390/ma10111304
Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications
Abstract
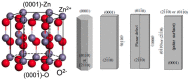
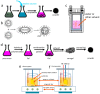
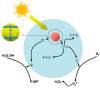
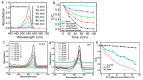
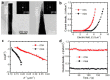
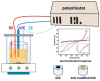
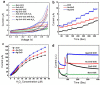
Zinc oxide (ZnO) nanostructures have been studied extensively in the past 20 years due to their novel electronic, photonic, mechanical and electrochemical properties. Recently, more attention has been paid to assemble nanoscale building blocks into three-dimensional (3D) complex hierarchical structures, which not only inherit the excellent properties of the single building blocks but also provide potential applications in the bottom-up fabrication of functional devices. This review article focuses on 3D ZnO hierarchical nanostructures, and summarizes major advances in the solution phase synthesis, applications in environment, and electrical/electrochemical devices. We present the principles and growth mechanisms of ZnO nanostructures via different solution methods, with an emphasis on rational control of the morphology and assembly. We then discuss the applications of 3D ZnO hierarchical nanostructures in photocatalysis, field emission, electrochemical sensor, and lithium ion batteries. Throughout the discussion, the relationship between the device performance and the microstructures of 3D ZnO hierarchical nanostructures will be highlighted. This review concludes with a personal perspective on the current challenges and future research.
Keywords: field emission; hierarchical nanostructures; lithium ion batteries; photocatalysis; sensor; solution phase synthesis; zinc oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Sun Y., Chen L., Bao Y., Zhang Y., Wang J., Fu M., Wu J., Ye D. The applications of morphology controlled ZnO in catalysis. Catalysts. 2016;6:188. doi: 10.3390/catal6120188. - DOI
-
- Wang Z.L. ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. R. 2009;64:33–71. doi: 10.1016/j.mser.2009.02.001. - DOI
-
- Wang Z.L. Nanostructures of zinc oxide. Mater. Today. 2004;7:26–33. doi: 10.1016/S1369-7021(04)00286-X. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

