A native-like bispecific antibody suppresses the inflammatory cytokine response by simultaneously neutralizing tumor necrosis factor-alpha and interleukin-17A
- PMID: 29137228
- PMCID: PMC5669854
- DOI: 10.18632/oncotarget.19899
A native-like bispecific antibody suppresses the inflammatory cytokine response by simultaneously neutralizing tumor necrosis factor-alpha and interleukin-17A
Abstract
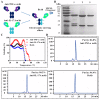
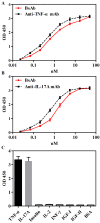
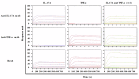
Anti-tumor necrosis factor (TNF) therapies are successful in the treatment of inflammatory disorders. However, some patients with rheumatoid arthritis (RA) fail to response anti-TNF drugs due to the compensation of other inflammatory signals. In this study, to reduce compensatory responses of interleukin-17A (IL-17A) during TNF-α inhibition, we generated an IgG-like bispecific antibodiy (bsAb) against TNF-α and IL-17A through a combination method of electrostatic Fc pairing and light chain crossover. This bsAb exhibited relatively high stability comparable to natural IgG antibodies, and retained the unaltered affinities to both of two targets. BsAb significantly decreased not only the expression level of neutrophil or Th17 chemokines, but also the secretion of IL-6/IL-8 on fibroblast-like synoviocytes (FLS) from a patient with RA. Meanwhile, TNF-α-mediated cellular cytotoxicity of fibroblasts was neutralized by bsAb. Importantly, we demonstrate that the combined blockade of TNF-α and IL-17A is more efficient than inhibition of either factor alone. Our results suggest the IgG-like anti-TNF-α/IL-17A bispecific molecule overcome the limited therapeutic responses using anti-TNF drugs. It may be a promising therapeutic agent for the treatment of autoimmune diseases.
Keywords: Fc heterodimerization; IL-17; Immune response; Immunity; Immunology and Microbiology Section; TNF-α; bispecific antibody; rheumatoid arthritis.
Conflict of interest statement
CONFLICTS OF INTEREST All authors have no potential conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

