NVP-BKM120 inhibits colon cancer growth via FoxO3a-dependent PUMA induction
- PMID: 29137323
- PMCID: PMC5669949
- DOI: 10.18632/oncotarget.20943
NVP-BKM120 inhibits colon cancer growth via FoxO3a-dependent PUMA induction
Abstract
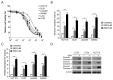
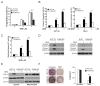
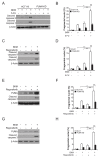
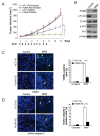
NVP-BKM120, a potent and highly selective PI3K inhibitor, is currently being investigated in phase I/II clinical trials. The mechanisms of action of NVP-BKM120 in colon cancer cells are unclear. In the present study, we investigated how NVP-BKM120 suppresses colon cancer cells growth and potentiates effects of other chemotherapeutic drugs. We found that NVP-BKM120 treatment enhance PUMA induction irrespective of p53 status through the FoxO3a pathway following AKT inhibition. Furthermore, PUMA is required for NVP-BKM120-induced apoptosis in colon cancer cells. In addition, NVP-BKM120 also synergized with 5-Fluorouracil or regorafenib to induce marked apoptosis via PUMA induction. Deficiency of PUMA suppressed apoptosis and antitumor effect of NVP-BKM120 in xenograft model. These results demonstrate a key role of PUMA in mediating the anticancer effects of NVP-BKM120 and suggest that PUMA could be used as an indicator of NVP-BKM120 sensitivity, and also have important implications for it clinical applications.
Keywords: FoxO3a; NVP-BKM120; PUMA; apoptosis; colon cancer.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


References
-
- Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. https://doi.org/10.1038/nrm3290. - DOI - PubMed
-
- Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–56. https://doi.org/10.1038/nrd4204. - DOI - PMC - PubMed
-
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. https://doi.org/10.1038/nrc2664. - DOI - PubMed
-
- Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer. 2010;10:342–52. https://doi.org/10.1038/nrc2842. - DOI - PubMed
-
- Speranza MC, Nowicki MO, Behera P, Cho CF, Chiocca EA, Lawler SE. BKM-120 (Buparlisib): a phosphatidyl-inositol-3 kinase inhibitor with anti-invasive properties in glioblastoma. Sci Rep. 2016;6:20189. https://doi.org/10.1038/srep20189. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

