Preoperative chronic kidney disease predicts poor oncological outcomes after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma
- PMID: 29137333
- PMCID: PMC5669959
- DOI: 10.18632/oncotarget.20554
Preoperative chronic kidney disease predicts poor oncological outcomes after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma
Abstract
Objective: To evaluate the impact of preoperative chronic kidney disease (CKD) on oncological outcomes in patients with upper tract urothelial carcinoma who underwent radical nephroureterectomy.
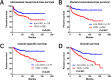
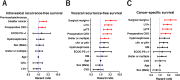
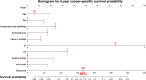
Methods: A total of 426 patients who underwent radical nephroureterectomy at five medical centers between February 1995 and February 2017 were retrospectively examined. Oncological outcomes, including intravesical recurrence-free, visceral recurrence-free, cancer-specific, and overall survival rates (intravesical RFS, visceral RFS, CSS, and OS, respectively) stratified by preoperative CKD status (CKD vs. non-CKD) were investigated. Cox proportional hazards regression analysis was performed using inverse probability of treatment weighting (IPTW) to evaluate the impact of preoperative CKD on prognosis and a prognostic factor-based risk stratification nomogram was developed.
Results: Of the 426 patients, 250 (59%) were diagnosed with CKD before radical nephroureterectomy. Before the background adjustment, intravesical RFS, visceral RFS, CSS, and OS after radical nephroureterectomy were significantly shorter in the CKD group than in the non-CKD group. Background-adjusted IPTW analysis demonstrated that preoperative CKD was significantly associated with poor visceral RFS, CSS, and OS after radical nephroureterectomy. Intravesical RFS was not significantly associated with preoperative CKD. The nomogram for predicting 5-year visceral RFS and CSS probability demonstrated a significant correlation with actual visceral RFS and CSS (c-index = 0.85 and 0.83, respectively).
Conclusions: Upper tract urothelial carcinoma patients with preoperative CKD had a significantly lower survival probability than those without CKD.
Keywords: chronic kidney disease; oncological outcome; radical nephroureterectomy; renal function; upper urinary tract urothelial carcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


References
-
- Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Bohle A, Van Rhijn BW, Kaasinen E, Palou J, Shariat SF. European Association of Urology Guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;68:868–79. https://doi.org/10.1016/j.eururo.2015.06.044. - DOI - PubMed
-
- Miyazaki J, Nishiyama H, Fujimoto H, Ohyama C, Koie T, Hinotsu S, Kikuchi E, Sakura M, Inokuchi J, Hara T. Laparoscopic versus open nephroureterectomy in muscle-invasive upper tract urothelial carcinoma: subanalysis of the multi-institutional national database of the Japanese Urological Association. J Endourol. 2016;30:520–5. https://doi.org/10.1089/end.2015.0757. - DOI - PubMed
-
- Lughezzani G, Burger M, Margulis V, Matin SF, Novara G, Roupret M, Shariat SF, Wood CG, Zigeuner R. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62:100–14. https://doi.org/10.1016/j.eururo.2012.02.030. - DOI - PubMed
-
- Mathieu R, Bensalah K, Lucca I, Mbeutcha A, Roupret M, Shariat SF. Upper urinary tract disease: what we know today and unmet needs. Transl Androl Urol. 2015;4:261–72. https://doi.org/10.3978/j.issn.2223-4683.2015.05.01. - DOI - PMC - PubMed
-
- Oya M, Kikuchi E. Evidenced-based clinical practice guideline for upper tract urothelial carcinoma (summary--Japanese Urological Association, 2014 edition) Int J Urol. 2015;22:3–13. https://doi.org/10.1111/iju.12630. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

