Long non-coding RNA SNHG15 inhibits P15 and KLF2 expression to promote pancreatic cancer proliferation through EZH2-mediated H3K27me3
- PMID: 29137412
- PMCID: PMC5663584
- DOI: 10.18632/oncotarget.20359
Long non-coding RNA SNHG15 inhibits P15 and KLF2 expression to promote pancreatic cancer proliferation through EZH2-mediated H3K27me3
Abstract
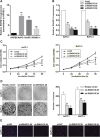
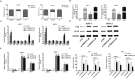
Long non-coding RNA (lncRNA) is emerging as an critical regulator in multiple cancers, including pancreatic cancer (PC). Recently, lncRNA SNHG15 was found to be up-regulated in gastric cancer and hepatocellular carcinoma, exerting oncogenic effects. Nevertheless, the biological function and regulatory mechanism of SNHG15 remain unclear in pancreatic cancer (PC). In this study, we reported that SNHG15 expression was also upregulated in PC tissues, and its overexpression was remarkably associated with tumor size, tumor node metastasis (TNM) stage and lymph node metastasis in patients with PC. SNHG15 knockdown inhibited proliferative capacities and suppressed apoptotic rate of PC cells in vitro, and impaired in-vivo tumorigenicity. Additionally, RNA immunoprecipitation (RIP) assays showed that SNHG15 epigenetically repressed the P15 and Kruppel-like factor 2 (KLF2) expression via binding to enhancer of zeste homolog 2 (EZH2), and chromatin immunoprecipitation assays (CHIP) assays demonstrated that EZH2 was capable of binding to promoter regions of P15 and KLF2 to induce histone H3 lysine 27 trimethylation (H3K27me3). Furthermore, rescue experiments indicated that SNHG15 oncogenic function partially involved P15 and KLF2 repression. Consistently, an inverse correlation between the expression of SNHG15 and traget genes were found in PC tissues. Our results reported that SNHG15 could act as an oncogene in PC, revealing its potential value as a biomarker for early detection and individualized therapy.
Keywords: P15 and KLF2; SNHG15; long noncoding RNA; pancreatic cancer; proliferation.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest were disclosed.
Figures





Similar articles
-
LINC01232 Promotes Gastric Cancer Proliferation through Interacting with EZH2 to Inhibit the Transcription of KLF2.J Microbiol Biotechnol. 2021 Oct 28;31(10):1358-1365. doi: 10.4014/jmb.2106.06041. J Microbiol Biotechnol. 2021. PMID: 34409953 Free PMC article.
-
Long non-coding RNA IRAIN suppresses apoptosis and promotes proliferation by binding to LSD1 and EZH2 in pancreatic cancer.Tumour Biol. 2016 Nov;37(11):14929-14937. doi: 10.1007/s13277-016-5380-8. Epub 2016 Sep 19. Tumour Biol. 2016. PMID: 27644252
-
Upregulated expression of long noncoding RNA SNHG15 promotes cell proliferation and invasion through regulates MMP2/MMP9 in patients with GC.Tumour Biol. 2016 May;37(5):6801-12. doi: 10.1007/s13277-015-4404-0. Epub 2015 Dec 10. Tumour Biol. 2016. PMID: 26662309
-
SNHG15: a promising cancer-related long noncoding RNA.Cancer Manag Res. 2019 Jul 1;11:5961-5969. doi: 10.2147/CMAR.S208054. eCollection 2019. Cancer Manag Res. 2019. PMID: 31308739 Free PMC article. Review.
-
LncRNA SNHG15: A new budding star in human cancers.Cell Prolif. 2020 Jan;53(1):e12716. doi: 10.1111/cpr.12716. Epub 2019 Nov 27. Cell Prolif. 2020. PMID: 31774607 Free PMC article.
Cited by
-
LINC00152 promotes pancreatic cancer cell proliferation, migration and invasion via targeting miR-150.Am J Transl Res. 2020 May 15;12(5):2241-2256. eCollection 2020. Am J Transl Res. 2020. PMID: 32509216 Free PMC article.
-
Application of Proteomics in Pancreatic Ductal Adenocarcinoma Biomarker Investigations: A Review.Int J Mol Sci. 2022 Feb 14;23(4):2093. doi: 10.3390/ijms23042093. Int J Mol Sci. 2022. PMID: 35216204 Free PMC article. Review.
-
PRC2 mediated KLF2 down regulation: a therapeutic and diagnostic axis during tumor progression.Cancer Cell Int. 2023 Oct 8;23(1):233. doi: 10.1186/s12935-023-03086-3. Cancer Cell Int. 2023. PMID: 37807067 Free PMC article. Review.
-
The multifaceted roles of long noncoding RNAs in pancreatic cancer: an update on what we know.Cancer Cell Int. 2020 Feb 5;20:41. doi: 10.1186/s12935-020-1126-1. eCollection 2020. Cancer Cell Int. 2020. PMID: 32042268 Free PMC article. Review.
-
Long noncoding RNA SNHG15: A promising target in human cancers.Front Oncol. 2023 Mar 28;13:1108564. doi: 10.3389/fonc.2023.1108564. eCollection 2023. Front Oncol. 2023. PMID: 37056344 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

